Abstract
Mercury (Hg) exposure, a worldwide public health concern, predominantly takes two forms – methylmercury from fish consumption and elemental Hg from dental amalgam restorations. We recruited 630 dental professionals from an American Dental Association meeting to assess Hg body burden and primary sources of exposure in a dually-exposed population. Participants described occupational practices and fish consumption patterns via questionnaire. Mercury levels in biomarkers of elemental Hg (urine) and methylmercury (hair, blood) were measured with a Direct Mercury Analyzer-80 and were higher than the general U.S. population. Geometric means (95% CI) were 1.28 (1.19–1.37) µg/L in urine, 0.60 (0.54–0.67) µg/g in hair, and 3.67 (3.38–3.98) µg/L in blood. In multivariable linear regression, personal amalgams predicted urine Hg levels along with total years in dentistry, amalgams handled, working hours, and sex. Fish consumption patterns predicted hair and blood Hg levels which were higher among Asians compared with Caucasians. Five species contributed the majority of the estimated Hg intake from fish - swordfish, fresh tuna, white canned tuna, whitefish, and king mackerel. When studying populations with occupational exposure to Hg, it is important to assess environmental exposures to both elemental Hg and methylmercury as these constitute a large proportion of total exposure.
Keywords: occupational exposure, environmental exposure, dentistry, fish consumption, mercury
1.0 INTRODUCTION
Mercury (Hg) is a toxic heavy metal that has been named the third priority pollutant of concern by U.S. regulatory bodies (1). The toxicity of Hg is complex and differs based on chemical speciation (2). Humans are primarily exposed to two species of Hg: methylmercury via fish consumption and inorganic Hg (as elemental Hg vapor) via dental amalgams (2,3). As such, exposures to Hg impact both the public and certain occupational groups (e.g., dentists, miners) worldwide. Hg exposure among dental professionals has been documented over time in the U.S. and has been declining due to changes in practice (4–7). Chronic low dose exposures to both methylmercury and elemental Hg is common. However, the health impact of the dual exposures is still not fully characterized, and studies often focus on only one Hg species.
The primary source of elemental Hg exposure for most people worldwide is through dental amalgam restorations which are comprised of approximately 50% elemental Hg (3). Overtime, Hg vaporizes, is absorbed in the lungs, and accumulates primarily in the kidneys following chronic exposure (2). Urinary excretion can reflect this form of Hg, especially when a significant source of elemental Hg exposure (such as amalgams) exists (8,9). Dental professionals exhibit higher levels of urinary Hg that correlate with their exposure from working with amalgam (7). Currently, the U.S. Food and Drug Administration considers dental amalgam to be a class II medical device (10). In general, Hg exposure from dental amalgam is considered safe (11). Dental professionals exhibit greater exposures to elemental Hg due to their practice, though the levels are much lower than that of other occupationally-exposed groups such as small-scale gold miners(12). The debate about safe levels of ambient occupational exposure to Hg vapor is ongoing (see review (13)). Furthermore, genetic susceptibility (see review (14)) and interaction with other toxicants (15) including methylmercury may also impact risk from chronic Hg exposure at relatively low doses.
Methylmercury is another Hg species of concern that the general public is exposed to via fish (2). Higher socioeconomic status and education are linked with greater fish consumption in countries such as the U.S., suggesting that dental professionals may have elevated exposures to methylmercury (16). Methylmercury risk assessment in the past has focused on neurodevelopmental impacts from in utero exposure as the primary public health concern (17). However, the impacts of methylmercury exposure in adulthood on cardiovascular and metabolic health outcomes are now becoming apparent (18–20). Risk-benefit balance and recommendations for safe fish consumption are complicated by the multitude of beneficial nutrients in fish that promote cardiovascular health (21), genetic susceptibility to toxicity from or accumulation of methylmercury (14), and interactions between exposure mixtures including chronic, low dose exposures to both Hg species.
While much is known about elemental Hg exposure among dental professionals, the extent of concurrent methylmercury exposure from fish consumption among this group is largely unknown. The purpose of this study was to increase understanding of exposures to both forms of Hg by conducting detailed exposure assessments and biomarker analyses. We recruited dental professionals from the American Dental Association (ADA, n=630) and collected information on two sources of Hg exposure. Due to their occupation and socioeconomic status, we hypothesized that the ADA participants would have elevated urine Hg concentrations (indicative of elemental Hg exposure) and hair and blood Hg concentrations (representative of methylmercury exposure) compared with the general U.S. population.
2.0 MATERIALS AND METHODS
2.1 Participant Recruitment
In October 2012, dental professionals (including dentists, dental hygienists or assistants, dental students, and office managers) attending the Health Screening Program at the ADA Annual Session in San Francisco, California were recruited to participate in this study. All participants provided written consent prior to participation. Institutional Review Board (IRB) approval was obtained from the University of Michigan (HUM00068339) and the ADA. The study aims were to determine sources of exposure to methylmercury and elemental Hg among dental professionals and to measure Hg biomarker levels. Individuals were allowed to participate even if they did not provide all biomarkers of interest or complete all questionnaires. Data were collected from 908 individuals, though only 630 subjects provided at least one biomarker (urine, hair, blood) for Hg analysis. Of these, 442 dental professionals provided extensive data on fish consumption patterns. This article details Hg exposure biomarkers and exposure sources, and thus includes available data from 630 dental professionals with Hg biomarkers.
Biospecimens were collected for laboratory analyses. Trained phlebotomists obtained blood samples (for Hg analyses) via venipuncture of the antecubital fossa into BD Vacutainer tubes certified for trace metals analysis. As previously described by us, hair was collected from the occipital region of the scalp (22). Single void spot urine samples were collected in metals-free containers at a random time of day (between 8 AM and 5 PM) similar to our previous collection and kept at 4°C until Hg analysis which occurred within one month of collection (6). Height, weight, and waist circumference were measured by trained health professionals.
2.2 Questionnaire Data
Participants completed two self-administered surveys detailing demographics (e.g., age, sex, race), occupational practices, smoking status, fish consumption patterns, and medical history. Sources of exposure to elemental Hg were evaluated (e.g., number of amalgams placed/removed per week, hours worked per week, years in dental practice, number of personal amalgams). Subjects reported fish consumption patterns over the past three months (consumption frequency, portion size, species). An estimated daily fish methylmercury intake value (µg/kg body weight/day) was calculated based on reported fish consumption and species-specific Hg concentrations reported by the U.S. Food and Drug Administration Monitoring Program in the most recent years available (see Supplemental Table 1) as previously described (22–27).
2.3 Hg Biomarkers
Total Hg levels were analyzed in whole blood (0.4 mL) and hair samples using a Direct Mercury Analyzer (DMA-80, Milestone Inc., Shelton, CT) according to U.S. EPA method 7473 as detailed by us (12,22). For hair Hg measurement, 5–10 mg of hair 2 cm long from the proximal end was used. Hair was washed with acetone, rinsed three times with Milli-Q water, and dried overnight prior to analysis. Quality control included running procedural blanks, sample replicates, and one certified reference material (CRMs; NIES CRM #13 for hair, INSPQ QMEQAS for blood, and DOLT-4 dogfish liver (Canadian National Research Council) as a general CRM) every ten samples. Mean recovery (±SD) of the CRMs was 95.6±6.7% for NIES hair, 99.8±10.6% for QMEQAS blood, and 100±5.9% for DOLT-4 dogfish liver. Analytical precision was assessed by running replicate CRM samples and ADA samples (every tenth sample). CRM replicates had good within-day (averages for the 3 CRMs ranged from 1.8–8.0% CV) and between-day (5.3–10.3% CV) agreement. Variability of replicate ADA hair and blood samples averaged 9.3% CV and 7.9% CV, respectively. The analytical detection limit, calculated as three times the SD of blank values was 0.09 ng Hg for hair and 0.23 µg/L for blood.
Urine Hg (2 mL) was measured via cold vapor atomic absorption spectroscopy at the ADA laboratory (6,7). Quality control at the ADA laboratory included running a procedural blank and a 2 µ/L Hg standard every 15 samples. No samples fell below the analytical detection limit of 0.025 µg/L. Specific gravity of urine samples was measured using a refractometer (PAL-10S, Atago U.S.A., Inc., WA). Urine Hg values were adjusted by specific gravity to decrease variability associated with spot urine sampling (28,29) according to the formula: [Adjusted Urine Hg = Urine Hg*(PSG/SG)] where PGS=(average ADA specific gravity – 1)*1000 and SG=(individual specific gravity – 1*1000). Specific gravity adjusted values are used unless otherwise indicated.
2.4 Statistical Analyses
All statistical analyses were performed using SAS v. 9.3 (SAS Institute Inc., Cary, N.C.). Tests were considered statistically significant when p-value <0.05. First, data distributions and univariate descriptive statistics were examined for all variables. Several implausible outliers were removed from the dataset (e.g., placing or removing >150 amalgams per week, >30 personal amalgams, consuming all 27 fish species multiple times per month). Hg biomarker data and estimated Hg from fish consumption were natural-log transformed to achieve normality, and the log-transformed values were used for analyses unless otherwise indicated. Statistical analyses included all subjects with the appropriate data for a given procedure, and as such sample sizes differ across analyses.
Relationships between Hg biomarkers and continuous demographic (e.g., age) or exposure source (e.g., years working, amalgams placed and removed, hours worked per week) variables were examined using Spearman Rank-Order Correlations for non-transformed data or Pearson correlations for log-transformed data. We performed ANOVA to compare Hg biomarker levels among categorical variables (sex, race, occupation, yes/no amalgam use questions). Welch’s ANOVA was used instead if variances were not homogenous according to Levene’s test.
Linear regression was used to determine the predictors of Hg biomarker levels. Hair, blood, or urine Hg (adjusted by average specific gravity (28,29)) were all natural log-transformed first. An automatic backward elimination method was employed to remove variables contributing the least to the model until only predictors with p<0.1 remained. All variables hypothesized to influence Hg exposure biomarker levels were originally included (amalgams, amalgams removed and placed, intake of Hg from fish consumption, occupation, sex, age, hours worked per week, smoking status (former/current vs. never smoker), BMI, race, and hemoglobin and red blood cell count (RBC; in blood Hg model)). Key hypothesized variables that were not selected in the elimination procedure were added one by one to the selected model, and variables with p<0.05 were retained in the final model. Age and total years working in the dental industry were highly correlated, and as such either one could be included in the final models with similar effect. In the hair and blood Hg models, age is included. Total years in dental practice is included in the urine Hg models. Final models were run in the total population and in dentists alone. An additional stratified analysis was run to statistically assess any cross-over of methylmercury exposure in urine or elemental Hg in hair and blood among individuals with low levels of reported exposure to the other species. The urine Hg model was run with and without fish Hg among individuals with small numbers of personal amalgams (<6) and/or occupationally handled amalgams (<20). Hair and blood Hg models were performed with and without amalgams and amalgams handled among subjects with limited fish consumption (<25th, <50th, and <75th percentiles of estimated fish Hg).
3.0 RESULTS
3.1 ADA Participant Characteristics
In 2012, 908 attendees of the ADA Annual Session provided samples and/or questionnaire data by participating in the Health Screening Program, and 630 of these participants provided sufficient data (e.g., at least one Hg biomarker) to be included here (see Table 1 for descriptive statistics). The ADA study population largely consisted of dentists (90.4%) and males (64.3%) that were middle-aged (mean±SD 54.8±11.4 years) though the age distribution was wide (range 21–82 years). Participants were primarily Caucasian (65.4%) or Asian (26.9%). Occupations differed among males and females. 98.2% of males were dentists compared to 76% of females of which 20.6% were dental hygienists or dental assistants (χ2 test p<0.0001 for occupational comparisons).
Table 1. Characteristics of American Dental Association (ADA) Study Participants.
| N1 | % | Mean ± SD | |
|---|---|---|---|
| Occupation | 603 | ||
| Dentists | 545 | 90.4 | |
| Dental Hygienists or Dental Assistants | 45 | 7.5 | |
| Other Dental-Related Professions | 13 | 2.2 | |
| Males | 396 | 64.3 | |
| Females | 220 | 35.7 | |
| Race2 | 592 | ||
| Caucasian | 387 | 65.4 | |
| Asian | 159 | 26.9 | |
| African American | 16 | 2.7 | |
| Smoking History | 438 | ||
| Never | 388 | 88.6 | |
| Past or Current | 50 | 11.4 | |
| Age (years) | 617 | 54.8 ±11.4 | |
| BMI (kg/m2) | 584 | 26.1 ± 5.0 | |
| Red Blood Cell Count (X 106 cells/µL) | 607 | 4.8 ± 0.4 | |
| Hemoglobin (g/dL) | 607 | 14.4 ± 1.3 | |
Not all subjects with Hg biomarkers (n=630) had complete datasets. Here, data presented for all subjects with each variable.
The remainder of participants (5.1%) self-identified with another race.
3.2 Hg Biomarker Levels
Total Hg concentrations were measured in hair, blood, and urine samples. Urine Hg values were adjusted by specific gravity to decrease variability from urinary dilution. Hg levels are presented in Table 2. Geometric means (GM, 95% CI) were 0.60 (0.54–0.67) µg/g for hair Hg, 3.67 (3.37–3.98) µg/L for blood Hg, and 1.28 (1.19–1.37) µg/L for unadjusted urine Hg. Hg levels ranged from 0.02 to 7.45 µg/g in hair, 0.16 to 25.3 µg/L in blood, and 0.13–13.1 µg/L in urine. The GM for the hair to blood Hg ratio was 165 (151–180).
Table 2. Mercury (Hg) Biomarker Levels.
Total Hg (non-speciated) in three biospecimens among participants of the American Dental Association 2012 study.
| N | Geometric Mean |
95% CI | Minimum | 25th Percentile |
50th Percentile |
75th Percentile |
Maximum | |
|---|---|---|---|---|---|---|---|---|
| Hair Hg (µg/g) | 424 | 0.60 | (0.54–0.67) | 0.01 | 0.33 | 0.69 | 1.27 | 7.45 |
| Blood Hg (µg/L) | 434 | 3.67 | (3.38–3.98) | 0.20 | 2.08 | 3.93 | 7.00 | 25.3 |
| Urine Hg (µg/L) | 606 | 1.28 | (1.19–1.37) | 0.13 | 0.71 | 1.31 | 2.48 | 13.1 |
| Urine Hg (µg/L)1 | 600 | 1.41 | (1.32–1.49) | 0.14 | 0.85 | 1.44 | 2.31 | 10.7 |
Adjusted by urinary specific gravity.
Hair and blood Hg levels, biomarkers of methylmercury exposure, were highly correlated (Pearson r=0.60, Figure 1A). Urine Hg was correlated with hair (r=0.29) and blood (r=0.31) Hg to a lesser yet statistically significant extent (Figures 1B and C). Hair and urine Hg levels were higher among dentists compared to dental hygienists and assistants or other occupations related to the dental profession (Table 3). Hg levels were also significantly higher in hair, blood, and urine of males compared with females (Table 3), and the sex-difference for urine Hg remained statistically significant (ANOVA p<0.0001) even when excluding non-dentists.
Figure 1. Correlation among Mercury (Hg) Biomarkers.
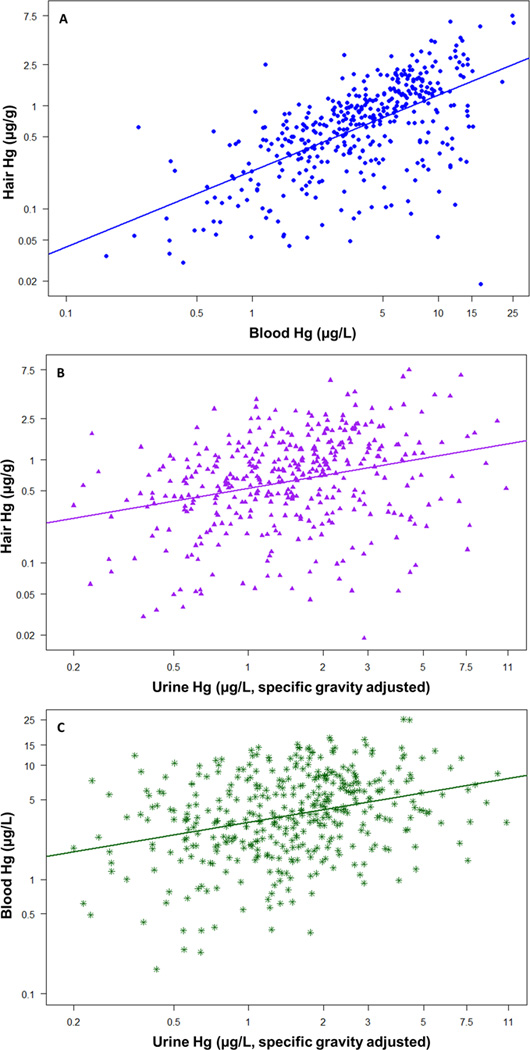
Hg concentrations in common biomarkers significantly correlated with one another (Pearson correlation test, p<0.05) among participants of the American Dental Association (ADA) study. Correlations between A) hair and blood (Pearson r=0.60, n=412), B) hair and urine (r=0.29, n=401), and C) blood and urine (r=0.31, n=407) Hg concentrations are depicted. To achieve normal distributions, variables were natural log-transformed first, but axes are labeled with standard values.
Table 3. Mercury (Hg) Levels across Occupational Groups and by Sex.
Urine Hg levels are specific gravity adjusted. Geometric means (GM) are reported for each group, and groups were compared by ANOVA of log-transformed variables. In the occupation comparison, 11 subjects with other occupations were excluded. Bold values indicate a significant ANOVA test.
| Dentists | Hygienists/Assistants | Males | Females | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | GM (95% CI) | N | GM (95% CI) | ANOVA p-value |
N | GM (95% CI) | N | GM (95% CI) | ANOVA p-value |
|
| Hair Hg (µg/g) | 369 | 0.62 (0.56–0.70) | 30 | 0.43 (0.30–0.60) | 0.06 | 257 | 0.66 (0.58–0.76) | 156 | 0.53 (0.46–0.62) | 0.04 |
| Blood Hg (µg/L) | 380 | 3.73 (3.42–4.07) | 29 | 2.85 (2.17–3.75) | 0.11 | 274 | 3.92 (3.53–4.36) | 158 | 3.30 (2.90–3.76) | 0.05 |
| Urine Hg (µg/L) | 522 | 1.52 (1.43–1.62) | 42 | 0.76 (0.60–0.97) | <0.0001 | 382 | 1.63 (1.52–1.75) | 205 | 1.08 (0.98–1.20) | <0.0001 |
3.3 Hg Exposure Sources
Details on sources of exposure to both elemental Hg via amalgams and methylmercury via fish consumption were collected with a questionnaire. Descriptive statistics for the exposure sources are presented in Table 4.
Table 4. Sources of Exposure to Elemental Mercury (Hg) and Methylmercury.
| N | Mean ± SD | Range | |
|---|---|---|---|
| Amalgams Placed/Assisted per week | 542 | 5.6 ± 10.9 | 0 – 100 |
| Amalgams Removed/Assisted per week | 542 | 8.4 ± 11.7 | 0 – 100 |
| Amalgams Handled per Week | 542 | 14.0 ± 19.4 | 0 – 200 |
| Posterior Composite Resin Restorations Placed/ Assisted per week |
540 | 10.7 ± 13.7 | 0 – 130 |
| Total Composite Resin Restorations Placed/ Assisted per week |
540 | 19.5 ± 19.0 | 0 – 150 |
| Total years in dental practice | 569 | 26.4 ± 11.1 | 0 – 55 |
| Hours Worked per week | 541 | 33.6 ± 8.7 | 0 – 60 |
| Amalgams in mouth | 436 | 3.7 ± 3.2 | 0 –15 |
| Hg intake from fish (µg/kg/day) | 412 | 0.10 (0.09–0.11)1 | 0 – 0.85 |
| Portion size at each fish meal (g) | 441 | 127 ± 69.7 | 0 – 340 |
| Fish Meals per Month | 441 | 10.2 (9.3–11.1)1 | 0 – 139 |
| Fish intake per month (kg) | 441 | 0.95 (0.80–1.13)1 | 0 – 35.4 |
| N2 | % Yes | ||
| In the past year, has the participant: | |||
| Had a mercury spill in current office | 11 | 1.8 | |
| Used amalgam | 340 | 56.9 | |
| Used only pre-capsulated amalgam | 353 | 59.0 | |
| Recycled amalgam scrap | 238 | 39.8 | |
| Recycled amalgam waste collected in filters/traps |
248 | 41.5 | |
| Used amalgam separators | 201 | 33.6 | |
Geometric mean (95% CI) reported instead due to distribution of the variable.
Number of participants who answered ‘yes’ is recorded. For all categories listed, 32 participants had missing data.
Elemental Hg
Occupational practices (e.g., working hours, use of amalgam or composite resin) varied widely among study subjects. Among dentists currently in practice (n=457), 84.9% remove or place Hg-containing amalgams (1–200 per week) though only 4.2% handle at least 50 amalgams per week. 8.3% of practicing dentists worked solely with composite resins, and 6.8% did not handle either composite resins or amalgams.
Spearman’s Rank-Order Correlation was used to examine relationships between non-transformed Hg biomarker levels (urine Hg was first adjusted for specific gravity) and elemental Hg exposure sources (see Supplemental Table 2). Handling amalgams in the occupational setting, number of amalgams in each individual’s mouth, and years working in the dental practice were positively correlated with urine Hg levels. Only years working was significantly correlated with blood or hair Hg. Likewise, urine, blood, and hair Hg levels increased with age (Spearman’s rho, ρ=0.24, p<0.0001; ρ =0.09, p=0.05; ρ =0.17, p=0.0007, respectively). As expected, total and posterior composite resins placed per week did not correlate with Hg biomarker levels. Urine Hg levels were greater among individuals who reported using amalgam or specifically pre-capsulated amalgam in their offices in the past year compared with professionals who did not (Supplemental Table 3, ANOVA tests). Recycling amalgam waste in traps or filters led to a near significant increase in urine Hg (p=0.07). No differences in Hg biomarker levels were observed based on use of amalgam separators or experiencing a Hg spill in the office in the previous year.
Methylmercury
Detailed data on fish consumption patterns of the past three months (e.g., frequency of consumption of 27 species of fish, portion size at each fish meal) was collected from 441 participants (Table 4, Supplemental Table 1). 95.2% of participants consumed fish in the preceding three months, and hair and blood Hg levels were significantly greater among those consuming fish (Welch’s ANOVA p<0.0001). Participants averaged 10.2 fish meals per month. An estimated Hg intake from fish consumption (µg/kg body weight/day) based on available Hg data in each fish species from U.S. databases (Supplemental Table 1) was calculated as we previously described (22,27). Supplemental Table 1 details average Hg levels assumed for each fish species, average consumption of each fish amongst the study participants, and average intake of Hg from each fish across study participants. Due to different concentrations of Hg in each fish species, the most commonly consumed fish (salmon, shrimp, white and light canned tuna, and tilapia) were not necessarily the species contributing the most to Hg intake (swordfish, fresh tuna, canned white tuna, king mackerel, and whitefish) in this group of dental professionals.
Correlations between non-transformed Hg biomarker levels and fish consumption variables were estimated using Spearman’s Rank-Order Correlation (see Supplemental Table 4). Estimated Hg intake from fish correlated well with increasing hair and blood Hg and weakly with urine Hg.
3.4 Predictors of Hg Biomarker Levels
Predictors of Hg biomarker levels were elucidated by running multivariable linear regression with available demographic, methylmercury and elemental Hg source variables (Table 5). The best models explained 25%, 23%, and 12% of the variation in urine Hg, hair Hg, and blood Hg, respectively.
Table 5. Predictors of Mercury (Hg) Biomarkers: Hair, Blood, and Urine.
Parameter estimates (SE) and their respective p-values (below, in bold if p<0.05) are reported for the best models predicting natural log-transformed biomarker levels. Urine Hg is specific gravity adjusted. Modeling non-adjusted urine Hg resulted in the same predictors with a worse fit (adjusted r2=0.18).
| Dependent | N | Adj. r2 | β0 | β1 | β2 | β3 | β4 | β5 |
|---|---|---|---|---|---|---|---|---|
| Hair Hg1 | 396 | 0.23 | −1.79 (0.23) | 0.02 (0.004) | 4.70 (0.46) | |||
| <0.0001 | 0.0002 | <0.0001 | ||||||
| Blood Hg2 | 404 | 0.12 | −0.33 (0.49) | 0.005 (0.004) | 2.79 (0.40) | 0.22 (0.09) | ||
| 0.50 | 0.14 | <0.0001 | 0.02 | |||||
| Urine Hg3 | 318 | 0.25 | −0.46 (0.20) | 0.01 (0.004) | −0.24 (0.08) | 0.08 (0.01) | 0.01 (0.004) | 0.005 (0.002) |
| 0.02 | 0.0009 | 0.004 | <0.0001 | 0.05 | 0.03 | |||
Hair Hg model: β0=intercept, β1= age (years), β2= estimated Hg intake from fish consumption (log-transformed, µg Hg/kg body weight/day)
Blood Hg model: β0=intercept, β1=age (years), β2=estimated Hg intake from fish consumption (log-transformed, µg Hg/kg body weight/day), β3=red blood cell count (X 106/µL)
Urine Hg model: β0=intercept, β1= total years in dental practice, β2=female (vs. male reference), β3=amalgams in mouth, β4=hours worked per week, β5=amalgams handled in the dental office per week (placed + removed)
Elemental Hg
Predictors of urine Hg levels were total years in dental practice, sex, number of amalgams in the subject’s mouth, hours worked per week, and number of amalgams placed and removed (‘handled’) in the dental office. Age, which is highly correlated with total years in dental practice (Spearman ρ=0.92), also predicted urine Hg levels. Total years working as a dental professional was included in the final model instead of age because 1) it led to a better model fit, and 2) it may serve as an indicator of number of years with elemental Hg exposure. When this model was run in the subset of dentists (n=302), the adjusted r2 was 0.24, and exhibited similar beta estimates and significance levels (though significance of sex and amalgams handled decreased slightly, p<0.1). The sample size varied considerably depending on which variables were included in the model. Hg levels and specific gravity were analyzed in 600 urine samples, but many subjects only provided data on a few variables of interest (e.g., years in dental profession, personal amalgams). Estimates for predictors were similar (e.g., direction and magnitude) but model fit decreased when urine Hg was not adjusted for specific gravity.
Initially, estimated Hg intake from fish (a source of methylmercury exposure) did not attain significance in the urine Hg models of the total population. We examined the contribution of fish Hg to urine Hg levels among subjects with limited sources of exposure to elemental Hg because previous isotope research suggested that some Hg in urine reflects methylmercury exposure (9). Among dental professionals with less than 6 personal amalgams (the 75th percentile), the same variables predicted urine Hg levels with an adjusted r2 of 0.19 (n=223), but Hg intake from fish consumption was an additional significant predictor and increased the adjusted r2 to 0.25. When excluding subjects in the highest quartile of personal amalgams (≥6) and/or of amalgams handled in the dental practice (≥20), fish Hg was a significant predictor of urine Hg levels. The inclusion of fish Hg increased the model adjusted r2 from 0.18 to 0.21 (n=167, data not shown).
Methylmercury
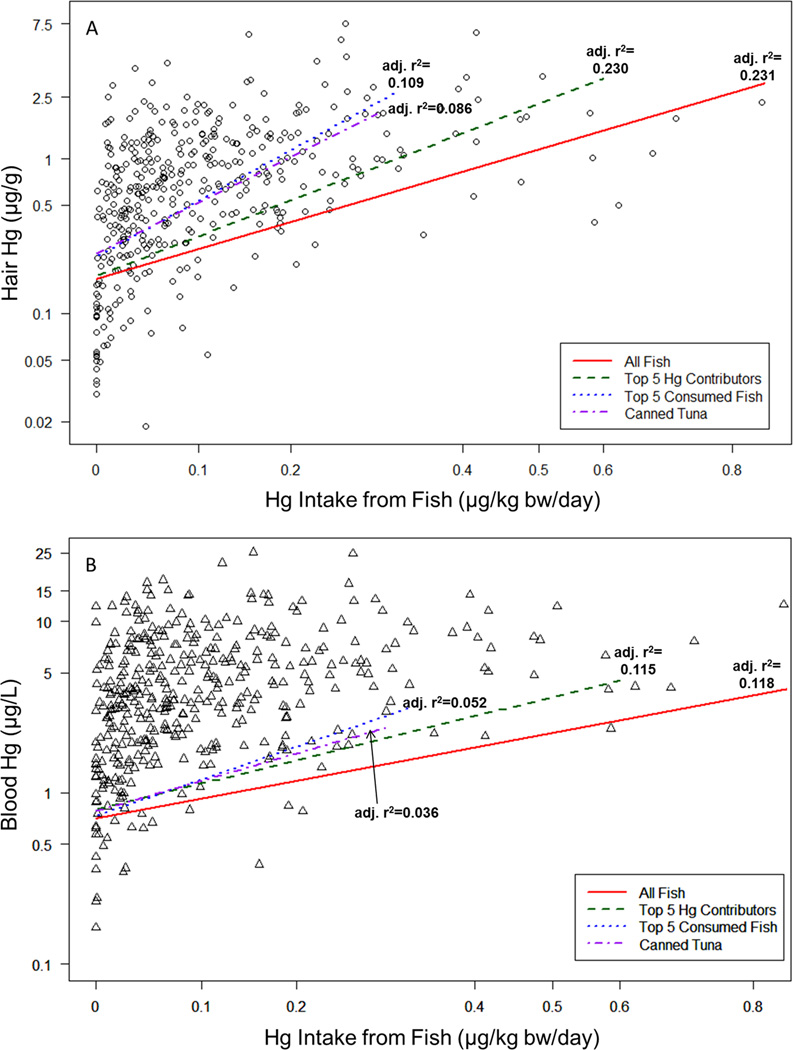
The best predictor of both hair and blood Hg was Hg intake from fish consumption, and levels also increased with age (Table 5). No elemental Hg exposure sources predicted increasing hair or blood Hg levels (though amalgams were negatively associated with hair Hg in some models). While either age or total years working in the dental practice predicted hair and blood Hg in a similar manner, age was included in the final models because workplace exposures did not predict hair and blood Hg levels. Blood Hg levels also increased with RBC count, and to a lesser extent, hemoglobin levels. When testing the models among subjects with <25th, <50th, or <75th percentiles of Hg intake from fish, fish Hg remained a strong predictor, and elemental Hg sources (amalgams, amalgams handled) did not predict hair or blood Hg concentrations (data not shown). When examining the contribution of individual fish species to hair and blood Hg levels, including only the five species providing the greatest portion of Hg intake (swordfish, fresh tuna, canned white tuna, king mackerel, whitefish) along with other model covariates predicted hair and blood Hg levels nearly as well as the original model including all 27 fish (>97% of the adjusted r2 for the main model, see Figure 2). When including only estimated Hg from the five most frequently consumed fish (salmon, shrimp, white canned tuna, tilapia, light canned tuna) instead for the fish Hg variable, the adjusted r2 was reduced to 0.052 and 0.109 for blood and hair Hg, respectively. Including only light and white canned tuna, two popularly consumed fish preparations, in the fish Hg intake variable resulted in models for blood and hair Hg with adjusted r2 of 0.036 and 0.086 (Figure 2).
Figure 2. Mercury (Hg) Intake and Hair and Blood Hg Levels.
Estimated Hg intake (µg Hg/ kg body weight/day) from reported fish consumption correlated with A) hair Hg and B) blood Hg levels. Regression lines representing relationships between fish Hg and hair Hg (adjusting for age) or blood Hg (adjusting for age and red blood cell count) are depicted for Hg intake estimated from 27 types of fish, the five fish contributing the greatest to Hg body burden, the five most frequently consumed fish, and canned tuna only. Lines end at the maximum intake from the respective group of fish. Model fit (adjusted r2) was the best for the models containing information based on all 27 fish species.
4.0 DISCUSSION
In this study, we characterized major sources of exposure to elemental Hg and methylmercury among dental professionals attending the Health Screening Program at the 2012 ADA Annual Session (n=630 participants). Dental professionals have occupational exposure to elemental Hg from dental amalgam restorations which consist of approximately 50% Hg (11). Furthermore, dental professionals are environmentally exposed to methylmercury via fish consumption, possibly more-so than the average population due to socioeconomic status (16), and elemental Hg via dental amalgams in their own mouths. These dental professionals exhibited higher hair, blood, and urine Hg levels (Table 2) compared to the U.S. population according to the NHANES (30,31), yet distributions overlapped considerably making this group relevant for the study of chronic low-dose exposure to two Hg species in the general population.
Over the last few decades, urine Hg levels monitored among ADA Annual Session attendees have demonstrated steady declines among dental professionals (5–7). Notably, reported arithmetic means decreased from 14.2 µg/L (average from 1975–1983) to 1.98 µg/L in 2012 (this study, dentists, unadjusted). Likewise, maximums >50 µg/L observed in the past were more than the maximum observed here (13.1 µg/L, unadjusted). The Hg levels we measured were similar to other recent studies among dental professionals in Michigan (GM 0.93 µg/L among the dentists, recruited in 2009–2010 (27)), Washington (2.5 µg/L among all recruited dentists in 1998 (32)), and West Scotland (2.58 nmol/mmol creatinine, recruited in early 2000s (33)) compared to 1.40 µg/L (GM, unadjusted, dentists only) in this study. While exposure differences among participants in each study (e.g., proportion of volunteers using amalgam, number of years in practice) may contribute to differences observed across studies, overall a trend for decreasing Hg levels among dental professionals is apparent. While 84.9% of working dentists in this study reported placing and removing amalgams, use may still be declining. Only 4.2% of dentists in this study remove or place >50 amalgams per week, and almost all professionals worked with both amalgam and composite resins. Lower urine Hg levels also likely reflect improvements in protective measures when working with Hg in amalgam (4). For example, among dentists handling amalgams, 74.7% reported using solely pre-capsulated amalgam, 52.1% recycle Hg waste collected in traps with vacuum pump filters, 42.1% use amalgam separators to prevent Hg particles from entering wastewater (34), and only 2.3% have experienced a Hg spill in the past year. Furthermore, >98% of dental professionals in this study wear gloves and face masks, though these protect better against infectious agents than Hg which is primarily inhaled in vapor form (2).
Future work with the ADA study may have relevance to the general U.S. population with regards to elemental Hg levels. The geometric mean in this dental population (unadjusted urine Hg) was nearly three times higher than that of U.S. adults enrolled in the 2007–2008 NHANES (0.48, 95% CI 0.44–0.52, n=1861 (31)) but distributions overlapped considerably. Furthermore, personal amalgams, an exposure source shared by the general population, explained a large portion of inter-individual urine Hg variance after adjustment for urinary specific gravity, sex, amalgams handled in the office, years in practice, and hours worked per week. An interquartile increase in number of personal amalgams predicts a 1.5 µg/L increase in urine Hg concentration compared with a 1.1 µg/L increase from either an interquartile shift in amalgams handled at the office, hours worked per week, or total years in the dental practice. Exposure sources predicting urine Hg levels (personal amalgams and occupational exposures) in this study were similar to that of other studies focused on dental professionals (7,27).
We observed significantly higher urine Hg levels among men even when excluding non-dentists and adjusting for exposure sources and BMI in multivariable linear regression. Sex differences in Hg levels have been observed in other dental populations (6,7,13). Toxicokinetic differences between the sexes may underlie differences in urinary Hg levels. Mouse studies suggest that inorganic Hg is more rapidly eliminated in males due to greater accumulation in the kidneys whereas more Hg is taken up by neurons in females (35–37). Thus, lower levels in females could reflect less urinary excretion and greater retention as opposed to a lower body burden overall compared with males.
Total Hg concentrations in hair and blood primarily reflect organic Hg (methylmercury) exposure (8). In the ADA study, we observed higher average hair and blood Hg levels compared to the general U.S. population, though distributions overlapped. This difference is likely due to fish consumption patterns as ADA participants consumed nearly double the amount of fish compared with NHANES adults (31.7 versus 17.3 grams per day, (38)). NHANES measured hair Hg in women and children recruited in 1999–2000. Hair Hg levels were on average 2.7 times higher among ADA females compared with the NHANES women (GM 0.53 µg/g vs. 0.2 µg/g (30)), and ADA males had significantly great hair Hg levels than the ADA females (GM 0.66 µg/g). Likewise, blood Hg levels among all ADA participants is 3.5 times higher than U.S. adults aged 20 and older measured as part of the 2009–2010 NHANES (GM 3.67 µg/L vs. 1.04 µg/L (31)). Males in the ADA study had higher hair and blood Hg levels than females, something we also observed in the Michigan Dental Association study (22).
A substantial proportion of ADA participants self-identified as Asian (26.9%), and this group had higher hair and blood Hg levels compared with other races. This difference can largely be explained by fish consumption patterns of the Asian participants. For example, based on our estimates, 37% of all subjects consumed levels of Hg in fish above the current U.S. EPA reference dose of 0.1µg/kg/day (39). Among Asians, 44% were above the reference dose. Additionally, 55% of all subjects had blood Hg levels >3.5 µg/L, a reference level set by the U.S. EPA, while 63% of Asians were over this mark. This reference level was designed to protect against neurodevelopmental impacts from prenatal exposure to methylmercury (40). While Hg levels in the ADA are many fold lower compared to at-risk groups such as populations subsisting on fish and sea mammals(41,42), hair Hg, and blood Hg levels in the study population are higher than other studies conducted in the U.S. including a study of women of child-bearing age conducted as part of the NHANES (43), and our previous study of dental professionals from the state of Michigan (22,27). Furthermore, among female ADA participants of childbearing age (<45 years), 60% of Asians and 25% of women from all other races exceeded the blood Hg reference level (3.5 µg/L). Cardiovascular and metabolic health impacts from methylmercury exposure are also of concern to this middle-aged group (18–20), and will be examined in future work. Fish consumption patterns may be influenced by region of residency, higher socioeconomic status, and race (Asian versus other), and these factors may be important for identifying subgroups from the broader population with greater exposure (16,44–47).
Hg concentrations in hair and blood were largely explained by fish consumption (Table 5). An interquartile increase in (log-transformed) estimated Hg intake from fish led to a 1.4 µg/L increase in blood Hg or a 1.7 µg/g increase in hair Hg. When considering the contribution of fish to Hg body burden, it is important to take into account both the amount of fish consumed and the species-specific Hg concentration. The most variability in blood and hair Hg levels was accounted for in models including Hg intake from 27 types of fish included in the survey. However, inclusion of five species contributing the most to Hg intake (swordfish, fresh tuna, white canned tuna, whitefish, and king mackerel) explained nearly as much variability as the model with all 27. Inclusion of only the top five most frequently consumed species (salmon, shrimp, white and light canned tuna, and tilapia) greatly reduced the amount of predicted variability in blood and hair Hg (<50% of full model, see Figure 2). Thus, detailed fish consumption patterns on multiple species, especially those known to have high Hg levels, are preferable to improve accuracy of exposure assessment compared with general measures of fish intake (e.g., total fish meals per month of any species). After accounting for Hg intake from fish, age remained positively associated with hair and blood Hg levels. This relationship has been observed in other studies (30,43,45) and may reflect accumulation over time or changes in Hg toxicokinetics with age. The majority of Hg in blood is found bound to proteins in RBCs such as hemoglobin. As such, inclusion of RBC count improved predictive capabilities in the blood Hg model to a greater extent than hemoglobin which has also been found to improve precision (48). Variability unaccounted for in hair and blood Hg models may reflect many factors including genetic differences in Hg toxicokinetics (see review (14)), consumption of fish species not included in the survey, and timing of fish meals, especially with regards to blood Hg which peaks for approximately one day following a fish meal (2).
The biomarkers selected for total Hg analysis in this study are commonly assumed to represent elemental Hg (urine) and methylmercury (hair, blood) exposure in the Hg literature (8). Multivariable analysis largely supported this assumption as fish consumption predicted hair and blood Hg levels while elemental Hg exposure sources (e.g., amalgams) did not. Urine Hg was predicted by elemental Hg exposure sources but not fish consumption in the total population. However, recent isotope work suggests that not all urine Hg is reflective of elemental Hg exposures; some reflects demethylated Hg from fish consumption (9). As such, we ran additional analyses in this study to model the contribution of fish Hg to urine Hg levels among subgroups with varying degrees of exposure to elemental Hg sources. These analyses demonstrated that fish Hg associated with urine Hg levels among subjects regularly exposed to less elemental Hg (e.g., individuals with <6 amalgams), and this should be taken into account in studies using urine Hg to represent elemental Hg exposure. Fish Hg remained the only Hg source of either species predicting hair and blood Hg levels even among subgroups with low levels of fish consumption (e.g., lowest quartile of fish Hg).
This study had many strengths including analysis of three Hg biomarkers and detailed reporting of exposure sources to both common Hg species: elemental Hg (from occupational practices and personal amalgams) and methylmercury (from consumption of 27 types of fish). Despite these strengths, this study was limited by the cross-sectional study design. Sources of Hg exposure were reported based on occupational practices in the past year, and fish consumption in the past three months. Likewise, Hg biomarker levels are reflective of recent exposures, though there is evidence that among individuals with consistent exposure (e.g., from a consistent fish consumption pattern or amalgams), a steady state level is achieved and hair or urine can reflect chronic exposure (3,49). A convenience sampling approach was used which could have resulted in recruitment of a non-representative group of dentists (with either higher or lower Hg exposure). However, dental professionals did not know their Hg levels before participating, and participants displayed varied exposures from personal amalgams (range 0–15), amalgams in the workplace (removing or placing 0–200 per week), and fish consumption (none to >1 fish meal per day) that enabled us to examine a range of Hg exposures that overlap with that of the U.S. population according to the NHANES. Spot urine samples were collected here (between 8AM and 5 PM) even though 24-hour urine collection is the gold-standard. While diurnal variation in urinary Hg levels has been observed in females, it is not typically found among males (50). To decrease variability from spot sampling, we adjusted urine metal concentrations for specific gravity (28,29).
In conclusion, we observed higher urine, hair, and blood Hg levels among dental professionals recruited at an ADA conference compared with the general U.S. population, and these biomarker levels were reflective of exposures from amalgams (urine) and fish consumption (hair, blood). Overall, urine Hg levels among dental professionals have decreased over the last several decades owing to changing practices to improve safety and health. When considering health implications of occupational exposures, it is also important to evaluate concurrent exposures from non-occupational sources (e.g., personal amalgams, fish) that contribute to Hg body burden and potentially to toxicity.
Supplementary Material
ACKNOWLEDGMENTS
We acknowledge the American Dental Association for their support. The University of Michigan (UM) field staff (Amanda Barks, Josillia Johnson, and Autumn Poisson) was instrumental in subject recruitment. Lara Khadr and Autumn Poisson were instrumental for Hg analysis, and Maxwell Scher and John Francis for specific gravity analysis. This research was supported by grants from the National Center for Advancing Translational Sciences of the National Institutes of Health (Award No. 2UL1TR000433), the UM Office of the Vice President for Research, the UM Environmental Health Sciences Core Center (Grant No. P30 ES017885), and the UM School of Public Health. JMG is also supported by U.S. Environmental Protection Agency (U.S. EPA) grants RD834800 and RD83543601 and National Institute for Environmental Health Sciences (NIEHS) grants P20 ES018171, P01 ES02284401. The contents of this publication are solely the responsibility of the grantee and do not necessarily represent the official views of the U.S. EPA or the NIH. Further, the U.S. EPA does not endorse the purchase of any commercial products or services mentioned in the publication. No conflict of interest is declared.
Footnotes
Supplemental Information is available at the Journal of Exposure Science and Environmental Epidemiology’s website.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
REFERENCES
- 1.ATSDR. [Accessed July 7, 2014];Priority List of Hazardous Substances. 2013 Available at: http://www.atsdr.cdc.gov/SPL/index.html.
- 2.Clarkson TW, Magos L. The toxicology of mercury and its chemical compounds. Critical Reviews in Toxicology. 2006;36(8):609–662. doi: 10.1080/10408440600845619. [DOI] [PubMed] [Google Scholar]
- 3.ATSDR. Toxicological profile for mercury. 1999 [Google Scholar]
- 4.ADA Council on Scientific Affairs. Dental mercury hygiene recommendations. J Am Dent Assoc. 2003;134(11):1498–1499. doi: 10.14219/jada.archive.2003.0081. [DOI] [PubMed] [Google Scholar]
- 5.Naleway C, Chou HN, Muller T, Dabney J, Roxe D, Siddiqui F. On-site screening for urinary Hg concentrations and correlation with glomerular and renal tubular function. J Public Health Dent. 1991;51(1):12–17. doi: 10.1111/j.1752-7325.1991.tb02169.x. [DOI] [PubMed] [Google Scholar]
- 6.Franzblau A, d’Arcy H, Ishak MB, Werner RA, Gillespie BW, Albers JW, et al. Low-level mercury exposure and peripheral nerve function. Neurotoxicology. 2012;33(3):299–306. doi: 10.1016/j.neuro.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 7.Martin MD, Naleway C, Chou HN. Factors contributing to mercury exposure in dentists. J Am Dent Assoc. 1995;126(11):1502–1511. doi: 10.14219/jada.archive.1995.0079. [DOI] [PubMed] [Google Scholar]
- 8.Berglund M, Lind B, Bjornberg KA, Palm B, Einarsson O, Vahter M. Inter-individual variations of human mercury exposure biomarkers: a cross-sectional assessment. Environ Health. 2005;4:20. doi: 10.1186/1476-069X-4-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sherman LS, Blum JD, Franzblau A, Basu N. New insight into biomarkers of human mercury exposure using naturally occurring mercury stable isotopes. Environ Sci Technol. 2013;47(7):3403–3409. doi: 10.1021/es305250z. [DOI] [PubMed] [Google Scholar]
- 10.U.S. Food and Drug Adminstration. [Accessed October 1, 2014];Appendix I: Summary of Changes to the Classification of Dental Amalgam and Mercury. 2009 Available at: http://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/DentalProducts/DentalAmalgam/ucm171120.htm.
- 11.Brownawell AM, Berent S, Brent RL, Bruckner JV, Doull J, Gershwin EM, et al. The potential adverse health effects of dental amalgam. Toxicol Rev. 2005;24(1):1–10. doi: 10.2165/00139709-200524010-00001. [DOI] [PubMed] [Google Scholar]
- 12.Paruchuri Y, Siuniak A, Johnson N, Levin E, Mitchell K, Goodrich JM, et al. Occupational and environmental mercury exposure among small-scale gold miners in the Talensi-Nabdam District of Ghana’s Upper East region. Sci Total Environ. 2010;408(24):6079–6085. doi: 10.1016/j.scitotenv.2010.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Richardson GM, Brecher RW, Scobie H, Hamblen J, Samuelian J, Smith C. Mercury vapour (Hg(0)): Continuing toxicological uncertainties, and establishing a Canadian reference exposure level. Regul Toxicol Pharmacol. 2009;53(1):32–38. doi: 10.1016/j.yrtph.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 14.Basu N, Goodrich JM, Head J. Ecogenetics of mercury: From genetic polymorphisms and epigenetics to risk assessment and decision-making. Environ Toxicol Chem. 2014;33(6):1248–1258. doi: 10.1002/etc.2375. [DOI] [PubMed] [Google Scholar]
- 15.Yorita Christensen KL, Carrico CK, Sanyal AJ, Gennings C. Multiple classes of environmental chemicals are associated with liver disease: NHANES 2003–2004. Int J Hyg Environ Health. 2013;216(6):703–709. doi: 10.1016/j.ijheh.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hightower JM, Moore D. Mercury levels in high-end consumers of fish. Environmental Health Perspectives. 2003;111:604–608. doi: 10.1289/ehp.5837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Trasande L, Landrigan PJ, Schechter C. Public health and economic consequences of methyl mercury toxicity to the developing brain. Environmental Health Perspectives. 2005;113(5):590–596. doi: 10.1289/ehp.7743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roman HA, Walsh TL, Coull BA, Dewailly E, Guallar E, Hattis D, et al. Evaluation of the cardiovascular effects of methylmercury exposures: current evidence supports development of a dose-response function for regulatory benefits analysis. Environ Health Perspect. 2011;119(5):607–614. doi: 10.1289/ehp.1003012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Salonen JT, Seppanen KFAU-, Lakka TAFAULT, Salonen RFAU, Kaplan GA, Kaplan GA. Mercury accumulation and accelerated progression of carotid atherosclerosis: a population-based prospective 4-year follow-up study in men in eastern Finland. Atherosclerosis. 2000;148(2):265–273. doi: 10.1016/s0021-9150(99)00272-5. [DOI] [PubMed] [Google Scholar]
- 20.He K, Xun P, Liu K, Morris S, Reis J, Guallar E. Mercury exposure in young adulthood and incidence of diabetes later in life: the CARDIA Trace Element Study. Diabetes Care. 2013;36(6):1584–1589. doi: 10.2337/dc12-1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cohen JT, Bellinger DC, Connor WE, Kris-Etherton PM, Lawrence RS, Savitz DA, et al. A quantitative risk-benefit analysis of changes in population fish consumption. Am J Prev Med. 2005;29(4):325–334. doi: 10.1016/j.amepre.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 22.Goodrich JM, Wang Y, Gillespie B, Werner R, Franzblau A, Basu N. Glutathione enzyme and selenoprotein polymorphisms associate with mercury biomarker levels in Michigan dental professionals. Toxicol Appl Pharmacol. 2011;257(2):301–308. doi: 10.1016/j.taap.2011.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.U.S. Food and Drug Administration. [Accessed December 11, 2013];Mercury Concentrations in Fish: FDA Monitoring Program. 1990–2010 Available at: http://www.fda.gov/Food/FoodborneIllnessContaminants/Metals/ucm191007.htm.
- 24.Bahnick D, Sauer C, Butterworth B, Kuehl DW. A national study of mercury contamination of fish: IV: Analytical methods and results. Chemosphere. 1994;29(3):537–546. [Google Scholar]
- 25.Mierzykowski SE, Carr KC. Total Mercury and Methyl Mercury in Freshwater Mussels (Elliptio complanata) from the Sudbury River Watershed, Massachusetts. USFWS. Spec. Proj. Rep. 2001 FY98-MEFO-2- EC. [Google Scholar]
- 26.EPA Gulf of Mexico Program. The Occurrence of Mercury in the Fishery Resources of the Gulf of Mexico, Stennis Space Center, MS. Public Health Focus Team. 2000 [Google Scholar]
- 27.Wang Y, Goodrich JM, Gillespie B, Werner R, Basu N, Franzblau A. An Investigation of Modifying Effects of Metallothionein Single Nucleotide Polymorphisms on the Association between Mercury Exposure and Biomarker Levels. Environ Health Perspect. 2012;120(4):530–534. doi: 10.1289/ehp.1104079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Levine L, Fahy JP. Evaluation of urinary lead determinations I, The significance of the specific gravity. J. Ind Hyg Toxicology. 1945;27:217. [Google Scholar]
- 29.Mason HJ, Calder IM. The correction of urinary mercury concentrations in untimed, random samples. Occup Environ Med. 1994;51(4):287. doi: 10.1136/oem.51.4.287-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McDowell MA, Dillon CF, Osterloh J, Bolger PM, Pellizzari E, Fernando R, et al. Hair mercury levels in U.S children and women of childbearing age: reference range data from NHANES 1999–2000. Environ Health Perspect. 2004;112(11):1165–1171. doi: 10.1289/ehp.7046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Centers for Disease Control and Prevention. Fourth National Report on Human Exposure to Environmental Chemicals, Updated Tables. 2013 Mar [Google Scholar]
- 32.Echeverria D, Woods JS, Heyer NJ, Rohlman D, Farin FM, Bittner AC, et al. Chronic low-level mercury exposure, BDNF polymorphism, and associations with cognitive and motor function. Neurotoxicol Teratol. 2005;27:781–796. doi: 10.1016/j.ntt.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 33.Ritchie KA, Burke FJT, Gilmour WH, Macdonald EB, Dale IM, Hamilton RM, et al. Mercury vapour levels in dental practices and body mercury levels of dentists and controls. British Dental Journal. 2004;197(10):625–632. doi: 10.1038/sj.bdj.4811831. [DOI] [PubMed] [Google Scholar]
- 34.Fan PL, Batchu H, Chou HN, Gasparac W, Sandrik J, Meyer DM. Laboratory evaluation of amalgam separators. J Am Dent Assoc. 2002;133(5):577–84. doi: 10.14219/jada.archive.2002.0233. [DOI] [PubMed] [Google Scholar]
- 35.Ekstrand J, Nielsen JB, Havarinasab S, Zalups RK, Soderkvist P, Hultman P. Mercury toxicokinetics--dependency on strain and gender. Toxicol Appl Pharmacol. 2009;243(3):283–91. doi: 10.1016/j.taap.2009.08.026. [DOI] [PubMed] [Google Scholar]
- 36.Nielsen JB. Toxicokinetics of mercuric chloride and methylmercuric chloride in mice. J Toxicol Environ Health. 1992;37(1):85–122. doi: 10.1080/15287399209531659. [DOI] [PubMed] [Google Scholar]
- 37.Pamphlett R, Ewan KB, McQuilty R, Waley P. Gender differences in the uptake of inorganic mercury by motor neurons. Neurotoxicol Teratol. 1997;19(4):287–293. doi: 10.1016/s0892-0362(97)00021-4. [DOI] [PubMed] [Google Scholar]
- 38.Papanikolaou Y, Brooks J, Reider C, Fulgoni VL., 3rd U.S adults are not meeting recommended levels for fish and omega-3 fatty acid intake: results of an analysis using observational data from NHANES 2003–2008. Nutr J. 2014;13:31. doi: 10.1186/1475-2891-13-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.U.S. Environmental Protection Agency. Water Quality Criterion for the Protection of Human Health: Methylmercury. EPA. 2001 823-R-01-001. [Google Scholar]
- 40.Stern AH, Smith AE. An assessment of the cord blood:maternal blood methylmercury ratio: implications for risk assessment. Environ Health Perspect. 2003;111(12):1465–1470. doi: 10.1289/ehp.6187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Choi AL, Weihe P, Budtz-Jorgensen E, Jorgensen PJ, Salonen JT, Tuomainen TP, et al. Methylmercury exposure and adverse cardiovascular effects in Faroese whaling men. Environ Health Perspect. 2009;117(3):367–372. doi: 10.1289/ehp.11608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Myers GJ, Davidson PW, Shamlaye CF, Axtell CD, Cernichiari E, Choisy O, et al. Effects of prenatal methylmercury exposure from a high fish diet on developmental milestones in the Seychelles Child Development Study. Neurotoxicology. 1997;18(3):819–829. [PubMed] [Google Scholar]
- 43.Mahaffey KR, Clickner RP, Bodurow CC. Blood organic mercury and dietary mercury intake: National Health and Nutrition Examination Survey, 1999 and 2000. Environ Health Perspect. 2004;112(5):562–570. doi: 10.1289/ehp.6587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hightower JM, O’Hare A, Hernandez GT. Blood mercury reporting in NHANES: identifying Asian, Pacific Islander, Native American, and multiracial groups. Environ Health Perspect. 2006;114(2):173–175. doi: 10.1289/ehp.8464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Park SK, Lee S, Basu N, Franzblau A. Associations of blood and urinary mercury with hypertension in U.S Adults: The NHANES 2003–2006. Environ Res. 2013;123:25–32. doi: 10.1016/j.envres.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Knobeloch L, Anderson HA, Imm P, Peters D, Smith A. Fish consumption, advisory awareness, and hair mercury levels among women of childbearing age. Environ Res. 2005;97(2):220–227. doi: 10.1016/j.envres.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 47.Mahaffey KR, Clickner RP, Jeffries RA. Adult women’s blood mercury concentrations vary regionally in the United States: association with patterns of fish consumption (NHANES 1999–2004) Environ Health Perspect. 2009;117(1):47–53. doi: 10.1289/ehp.11674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim BM, Choi AL, Ha EH, Pedersen L, Nielsen F, Weihe P, et al. Effect of hemoglobin adjustment on the precision of mercury concentrations in maternal and cord blood. Environ Res. 2014;132:407–412. doi: 10.1016/j.envres.2014.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bartell SM, Ponce RA, Sanga RN, Faustman EM. Human variability in mercury toxicokinetics and steady state biomarker ratios. Environ Res. 2000;84(2):127–132. doi: 10.1006/enrs.2000.4104. [DOI] [PubMed] [Google Scholar]
- 50.Woods JS, Martin MD, Leroux BG. Validity of spot urine samples as a surrogate measure of 24-hour porphyrin excretion rates Evaluation of diurnal variations in porphyrin, mercury, and creatinine concentrations among subjects with very low occupational mercury exposure. J Occup Environ Med. 1998;40(12):1090–1101. doi: 10.1097/00043764-199812000-00009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.