Abstract
AIM: To elucidate the impact of various donor recipient and transplant factors on the development of biliary complications after liver transplantation.
METHODS: We retrospectively reviewed 200 patients of our newly established liver transplantation (LT) program, who received full size liver graft. Biliary reconstruction was performed by side-to-side (SS), end-to-end (EE) anastomosis or hepeaticojejunostomy (HJ). Biliary complications (BC), anastomotic stenosis, bile leak, papillary stenosis, biliary drain complication, ischemic type biliary lesion (ITBL) were evaluated by studying patient records, corresponding radiologic imaging and reports of interventional procedures [e.g., endoscopic retrograde cholangiopancreatography (ERCP)]. Laboratory results included alanine aminotransferase (ALT), gammaglutamyltransferase and direct/indirect bilirubin with focus on the first and fifth postoperative day, six weeks after LT. The routinely employed external bile drain was examined by a routine cholangiography on the fifth postoperative day and six weeks after transplantation as a standard procedure, but also whenever clinically indicated. If necessary, interventional (e.g., ERCP) or surgical therapy was performed. In case of biliary complication, patients were selected, assigned to different complication-groups and subsequently reviewed in detail. To evaluate the patients outcome, we focussed on appearance of postoperative/post-interventional cholangitis, need for rehospitalisation, retransplantation, ITBL or death caused by BC.
RESULTS: A total of 200 patients [age: 56 (19-72), alcoholic cirrhosis: n = 64 (32%), hepatocellular carcinoma: n = 40 (20%), acute liver failure: n = 23 (11.5%), cryptogenic cirrhosis: n = 22 (11%), hepatitis B virus /hepatitis C virus cirrhosis: n = 13 (6.5%), primary sclerosing cholangitis: n = 13 (6.5%), others: n = 25 (12.5%) were included. The median follow-up was 27 mo until June 2015. The overall biliary complication rate was 37.5% (n = 75) with anastomotic strictures (AS): n = 38 (19%), bile leak (BL): n = 12 (6%), biliary drain complication: n = 12 (6%); papillary stenosis (PS): n = 7 (3.5%), ITBL: n = 6 (3%). Clinically relevant were only 19% (n = 38). We established a comprehensive classification for AS with four grades according to clinical relevance. The reconstruction techniques [SS: n = 164, EE: n = 18, HJ: n = 18] showed no significant impact on the development of BCs in general (all n < 0.05), whereas in the HJ group significantly less AS were found (P = 0.031). The length of donor intensive care unit stay over 6 d had a significant influence on BC development (P = 0.007, HR = 2.85; 95%CI: 1.33-6.08) in the binary logistic regression model, whereas other reviewed variables had not [warm ischemic time > 45 min (P = 0.543), cold ischemic time > 10 h (P = 0.114), ALT init > 1500 U/L (P = 0.631), bilirubin init > 5 mg/dL (P = 0.595), donor age > 65 (P = 0.244), donor sex (P = 0.068), rescue organ (P = 0.971)]. 13% (n = 10) of BCs had no therapeutic consequences, 36% (n = 27) resulted in repeated lab control, 40% (n = 30) received ERCP and 11% (n = 8) surgical therapy. Fifteen (7.5%) patients developed cholangitis [AS (n = 6), ITBL (n = 5), PS (n = 3), biliary lesion BL (n = 1)]. One patient developed ITBL twelve months after LT and subsequently needed retransplantation. Rehospitalisation rate was 10.5 % (n= 21) [AS (n = 11), ITBL (n = 5), PS (n = 3), BL (n = 1)] with intervention or reinterventional therapy as main reasons. Retransplantation was performed in 5 (2.5%) patients [ITBL (n = 1), acute liver injury (ALI) by organ rejection (n = 3), ALI by occlusion of hepatic artery (n = 1)]. In total 21 (10.5%) patients died within the follow-up period. Out of these, one patient with AS developed severe fatal chologenic sepsis after ERCP.
CONCLUSION: In our data biliary reconstruction technique and ischemic times seem to have little impact on the development of BCs.
Keywords: Liver transplantation, Biliary complications, Anastomotic stenosis, Ischemic type biliary lesion, Non-anastomotic strictures, Bile leak, Ischemic time, Biliary drain complications
Core tip: This study evaluates the impact of various factors on development of biliary complications (BC) after liver transplantation (LT). Biliary reconstruction technique and ischemic times, as well as other donor- and recipient- factors did not influence appearance of BC. However, length of donor-intensive care unit-stay over 6 d did. Furthermore we are the first to describe a comprehensive classification of anastomotic strictures after LT according to clinical relevance.
INTRODUCTION
Liver transplantation (LT) is currently the standard therapeutic procedure for patients with end-stage liver disease. Over the last decades, surgical techniques, immunosuppression and postoperative management have improved constantly resulting in better patient outcome. Nevertheless biliary strictures and leakages still belong to the most frequent complications after liver transplantation with an incidence of 10%-35%[1-3]. Biliary complications (BC) are associated with significantly higher morbidity and mortality rates (2%-7%)[4,5]. This often results in frequent reinterventions, hospital readmissions, and thus higher costs. Furthermore they can lead to acute and/or chronic liver injury[1-3,6].
The range of complications within the biliary tract is relatively wide and includes anastomotic strictures (AS), non-anastomotic strictures (NAS), papillary dysfunction/stenosis and bile leaks with anastomotic strictures and bile leaks being the most frequent[7-10].
An anastomotic stricture is defined as narrowing of the anastomosis between the recipient and the donor bile ducts. It typically occurs within the first six months[7,11] but clinical manifestation years after LT is also possible[11,12]. The majority of anastomotic stenoses (60%-90%) remains asymptomatic or can be treated by endoscopic retrograde cholangiography (ERCP) with interventional dilatation and/or stenting[13], whereas 10%-20% of patients need surgical intervention[14,15].
NAS may be found at any site of the biliary tree (extra- or intrahepatic). The incidence ranges in different studies from 1%-20% and occurs only in 50% within the first year after related injury due to LT. NAS occurring within the first year (early onset) is suggested to be associated with ischemia to hepatic artery thrombosis (HAT), but it can also occur without HAT so called “ischemic type biliary lesion” (ITBL). On the other hand NAS occurring within patients course is probably caused by immunological factors[16,17]. In contrast to the AS this disease pattern is not easy to handle and has a high rate of morbidity and mortality[15]. Next to anastomotic strictures, bile leakages are reported after full-size LT in about 1%-25%[1,18]. They often appear in the early postoperative period and can most often be localized easily. The use of a T-tube in duct-to-duct (DD) biliary reconstruction is still under debate. While older series[19,20] report leakages or complications after removal of the T-tube at the site of insertion with frequency up to 33%, a more recent randomized controlled trial clearly favours T-tube insertion for side-to-side (SS) biliary reconstruction in deceased donor liver transplantation (DDLT)[21]. Overall the incidence of biliary complications in DDLT is dependent on a variety of concurrent factors, such as the type of liver transplant procedure, organ preservation, hepatic artery thrombosis, use of an external or internal drainage of bile duct anastomosis, ischemia/reperfusion injury, immunological and other specific donor and recipient characteristics[22]. The type of biliary reconstruction plays a major role.
Choledochocholedochostomy (CC) can be performed in end-to-end (EE) or SS technique. Hepaticojejunostomy (HJ) with a Roux-en-y loop reconstruction is commonly used in cases of pre-existing biliary disease [e.g., primary sclerosing cholangitis (PSC)]or if DD reconstruction is not possible[23].
The decision which technique has to be employed, therefore depends on the patient’s primary indication, the possible difference in size between recipient and donor bile duct and possible prior biliary surgery.
The present study analyses our experiences with the first 200 patients of our recently established liver transplant centre. Special respect is paid to the impact of the reconstruction technique and ischemic time as well as donor organ quality.
MATERIALS AND METHODS
Between May 2010 and March 2015 a total number of 228 liver transplantations were performed in our centre. Twenty-eight patients were not eligible for study inclusion for various reasons (early death/lost to follow up). In this study we retrospectively reviewed the records of 200 patients who received a deceased full size liver graft. No AB0 incompatible grafts were transplanted. The median follow-up was 27 mo until June 2015. Recipient and donor characteristics are shown in Tables 1 and 2.
Table 1.
Recipient characteristics
| Parameters | n (%) |
| Age | 56 (19-72) |
| Gender | |
| Male | 135 (67.5) |
| Female | 65 (32.5) |
| Indication for LT | |
| Alcoholic cirrhosis | 64 (32) |
| HCC | 40 (20) |
| Acute liver failure | 23 (11.5) |
| Cryptogenic cirrhosis | 22 (11) |
| HBV/HCV cirrhosis | 13 (6.5) |
| PSC | 13 (6.5) |
| Others | 25 (12.5) |
LT: Liver transplantation; HCC: Hepatocellular carcinoma; HBV/HCV: Hepatitis B/C virus; PSC: Primary sclerosing cholangitis.
Table 2.
Donor characteristics
| Parameters | |
| Age, yr | 56 (12-89) |
| Gender, n (%) | |
| Male | 98 (49) |
| Female | 102 (51) |
| ICU, d | 3 (0-60) |
| BW, kg | 84.5 (30-190) |
ICU: Intensive care unit; BW: Bodyweight.
Biliary complications were evaluated by studying patient records (discharge letters, surgical reports/donor reports and laboratory results), corresponding radiologic imaging especially magnetic resonance tomography/magnet resonance cholangiopancreatography and reports of interventional procedures (e.g., ERCP). In case of biliary complication, patients were selected, assigned to different complication-groups and subsequently reviewed in detail.
Laboratory results were obtained from the medical database of the Aachen University Hospital. Analysed data were aspartate aminotransferase, alanine aminotransferase (ALT), gamma glutamyltransferase (GGT) and direct/indirect bilirubin. We focused on the results of the first and fifth postoperative day, six weeks after LT and on laboratory results in cases of biliary complication at the time of diagnosis.
Transplant procedure
We used an extracorporeal venovenous/portalvenous bypass in every LT procedure. The transplantation was performed starting with the anastomosis of the suprahepatic vena cava (VC), followed by the infrahepatic VC and the hepatic artery. A portal venous EE anastomosis was performed before the simultaneous arterial and portal venous reperfusion. We routinely perform a CC in form of a SS anastomosis. In patients who have to be transplanted because of a PSC, a HJ was performed for biliary reconstruction. We also prefer to place an external biliary drain (T-tube/Roeder-drain). Transplant characteristics are depicted in Table 3.
Table 3.
Transplantation data
| Parameters | |
| WIT, min | 44 (20-78) |
| CIT, min | 480 (100-994) |
| Rescue allocation | 93 (46.5) |
| Anastomotic technique | |
| SS | 164 (82) |
| EE | 18 (9) |
| Hepaticojejunostomy | 18 (9) |
| External biliary drain | |
| T-tube | 179 (89.5) |
| Roeder-drain | 15 (7.5) |
| No drain | 6 (3) |
CIT: Cold ischemic time; WIT: Warm ischemic time; SS: Side-to-side; EE:End-to-end.
Routine imaging and handling of the T-tube
The external bile drain is examined by a routine cholangiography on the fifth postoperative day and six weeks after transplantation as a standard procedure, but also whenever clinically indicated.
If the postoperative course was uneventful, the demonstration showed no pathologies with a sufficient outflow of contrast medium into the duodenum and bilirubin levels deceased permanently, the T-tube was clamped. This was followed by control of the laboratory results to exclude increasing bilirubin levels or cholestatic parameters afterwards.
Six weeks after LT a routine terminal X-ray cholangiography took place and the T-tube was removed in case of normal clinical and radiological settings.
Definition of complications
Anastomotic strictures were defined by X-ray cholangiography or ERCP as a focal or segmental narrowing at the site of biliary anastomosis. They were accompanied by good, delayed or absent bile efflux to the intestinal tract and with or without cholestatic signs. Patients with unessential changes in calibre, or with signs of anastomotic narrowing only on the fifth postoperative day without cholestatic lab parameters, were not defined as a stricture (Examples are shown by Figures 1 and 2).
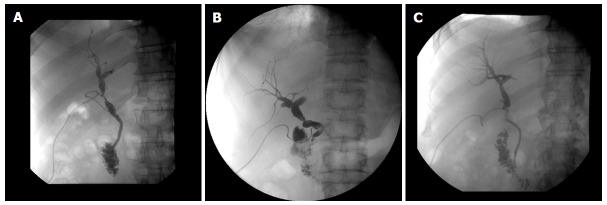
Figure 1.
Normal anatomy of bile duct anastomosis (side-to-side): T-tube X-ray six weeks after liver transplantation.
Figure 2.
Different types of bile duct anastomotic pathologies: All T-tube X-rays six weeks after liver transplantation. A: Stenosis (> 30%) after side-to-side anstomosis, resolved after endoscopic stent treatment for 3 mo; B: Stenosis (> 30%) after end-to-end anstomosis, all lab values normal, no clinical relevance, no intervention; C: No anastomotic stenosis but incongruence of graft- and recipient bile duct, no clinical relevance, normal lab values, surveillance.
To our best knowledge there is no widely accepted classification of AS described so far. Thus we divided anastomotic strictures depending on laboratory results and clinical pattern into four grades:
Grade 1: Segmental narrowing in X-ray cholangiography or ERCP (< 30%), no clinical symptoms, no cholestatic parameters (GGT/bilirubin)
Grade 2: Segmental narrowing in X-ray cholangiography or ERCP (> 30%), no clinical symptoms, no bilirubin, increased GGT
Grade 3: Segmental narrowing in X-ray cholangiography or ERCP, no clinical symptoms, increased bilirubin and GGT
Grade 4: Segmental narrowing in X-ray cholangiography or ERCP and clinical symptoms (cholangitis, jaundice)
Bile leaks were defined by emission of contrast medium seen in the X-ray cholangiography or by bile secretion seen in the abdominal drains.
Papillary stenosis was defined by prepapillary bile duct dilatation with mainly delayed bile efflux by X-ray cholangiography or ERCP.
Complications of biliary drain were defined by X-ray cholangiography in form of displacement into the intestinal tract or the abdominal cavity as well as other rare clinical manifestations (e.g., rupture by removal).
Ischemic type biliary lesions were diagnosed by pathological lab values, endoscopic retrograde cholangiography and were characterized by non-anastomotic strictures in the absence of a hepatic artery thrombosis.
Treatment
Different biliary complications require different therapeutic strategies according to the clinical aspect of the patient and the medical “hard facts” (laboratory results, radiological imaging). Accordingly we categorized the type of therapy into four main groups: 0. No therapy needed; 1. Repeated control of the laboratory results (no intervention); 2. Intervention needed [ERCP/percutane transhepatic cholangiodrainage (PTCD)]; 3. Operative therapy.
Patients who did not show clinical symptoms nor pathological lab values or clearly pathological X-ray results did not need any therapy. Interventional therapy was mainly performed as ERCP, which includes technical details like sphincterotomy, dilatation and implantation of bile duct stents, if needed. In most cases intervention was successful, but sometimes sequential ERCPs were necessary to achieve adequate results. In some patients with hepaticojejunostomy PTCD procedures were performed.
If surgery was required, operative procedures included early revisions with re-sewing of bile leaks or performing a HJ if the latter was impossible, or late retransplantation for ITBL.
Outcome
We focussed on the appearance of postoperative/post-interventional cholangitis, the need of rehospitalisation, need of retransplantation, incidence of ITBL and death caused by BC.
Statistical analysis
Continuous data are expressed as median and range (X, Y-Z), or mean ± SD. Categorical variables were compared by the χ2-test. Furthermore categorical variables were analysed using a binary logistic regression model to estimate their impact on development of biliary complications. A P-value < 0.05 was considered statistically significant. All calculations were done using the SPSS software package (version 23.0 for Windows, SPSS, Inc., Chicago, IL).
RESULTS
Two hundred patients undergoing liver transplantation at the University Hospital Aachen between 2010 and 2015 were studied retrospectively in detail.
Recipient characteristics
The median age was 56 (19-72) years. The male to female ratio 135:65. The main reasons for liver transplantation were hepatocellular carcinoma (HCC) and alcoholic induced liver cirrhosis. The demographics of recipient patients are shown in Table 1.
Donor characteristics
The median age was 56 (12-89) years. Male-to-female ratio was 98:102 with a median bodyweight of 84.5 kg (30-190). Demographics of donors are shown in Table 2.
Transplantation data
For biliary reconstruction we performed a SS CC in 82% of the patients. Percent of 9 received a HJ and 9% an EE reconstruction. The type of reconstruction was dependent of the primary indication for LT and anatomical conditions.
An external biliary drain (T-tube/Roeder-drain) was placed in 194 patients during reconstruction procedure. In six patients we disclaimed any external biliary drain, due to technical difficulties.
The median warm ischemic time was 44 min (20-78). The median cold ischemic time was 480 min (100-994). Percent of 47 of the transplanted organs were allocated by a rescue-allocation procedure (“marginal organs”). The transplantation data are shown in Table 3.
Biliary complications in relation to the type of biliary reconstruction technique
Biliary complications are summarized in Table 4. These are divided according to the time of appearance into early (within the first three months after LT) and late onset (after three months). In total in 37% (n = 75) of the 200 liver transplanted patients biliary complications were found. Of these patients only 40% (n = 30) needed interventional therapy and 11% (n = 8) underwent surgical therapy.
Table 4.
Biliary complications in relation to the type of biliary reconstruction technique n (%)
| SS n = 164 | P-vaule (vs not SS) | EE n = 18 | P-vaule (vs not EE) | HJ n = 18 | P-vaule (vs not HJ) | |
| Anastomotic strictures | 34 (20.75) | 0.183 | 4 (22.2) | 0.715 | 0 | 0.031 |
| < 3 mo | 29 (17.7) | 2 (11.1) | ||||
| > 3 mo | 5 (3.05) | 2 (11.1) | ||||
| Bile leaks | 10 (6.1) | 0.901 | 0 | 0.261 | 2 (11.1) | 0.338 |
| Biliary drain complications | 10 (6.1) | 0.091 | 0 | 0.261 | 2 (11.1) | 0.338 |
| Papillary stenosis | 7 (4.3) | 0.207 | 0 | 0.397 | - | |
| ITBL | 6 (3.6) | 0.244 | 0 | 0.434 | 0 | 0.434 |
| ≤ 1st yr | 4 (2.4) | |||||
| > 1st yr | 2 (1.2) | |||||
| Total | 67 (40.9) | 4 (22.2) | 4 (22.2) |
SS: Side-to side; EE: End-to-end; HJ: Hepeaticojejunostomy; ITBL: Ischemic type biliary lesion.
In patients who received a SS bile duct anastomosis 34 (21%) of the 164 patients had an AS (18% early onset, 3% late onset). In the group of patients with an EE anastomosis, 4 (22%) of 18 developed an AS (11% early onset, 11% late onset) and therefore showed no significant difference compared to the other reconstruction techniques (SS, HJ). The group with a HJ reconstruction showed no anastomotic stricture at all. Compared to the other reconstructive procedures this was statistically significant (P = 0.031).
Bile leaks and biliary drain complication both occurred in ten (6%) of 164 patients in the group of SS-anastomosis within the first three months. The EE-group had none of these. Patients with biliary reconstruction by HJ showed two (11%) bile leaks and two (11%) biliary drain complications within the first three months. Papillary stenosis was seen in seven (4.3%) and ITBL in six (3.6%) of 164 SS-anastomoses (2.4% within the first year, 1.2% after one year).
General biliary complications in relation to ischemic times, initial postoperative lab-values and specific donor data
In addition to the type of biliary reconstruction technique we reviewed several other variables to identify possible predictors for biliary complications. Those are warm ischemic time (WIT), cold ischemic time (CIT), initial ALT and bilirubin lab results measured on the first postoperative day, as well as donor age, donor sex, length of donor intensive care unit (ICU) stay (d) and rescue allocation. As shown in Table 5, none of these variables seemed to influence the incidence of BCs, whereas length of donor ICU stay above six days was significantly more frequent in recipients suffering from BCs (P = 0.007).
Table 5.
Biliary complications in relation to ischemic times, initial postoperative lab-values and specific donor data n (%)
| BC yes | BC no | P-value (χ2) | |
| WIT > 45 min | 29 (38.7) | 43 (34.4) | 0.543 |
| CIT > 10 h | 10 (13.3) | 28 (22.4) | 0.114 |
| ALT init | 9 (12) | 18 (14.4) | 0.631 |
| > 1500 U/L | |||
| Bilirubin init | 19 (25.3) | 36 (28.8) | 0.595 |
| > 5 mg/dL | |||
| Donor Age | 23 (30.7) | 29 (23.2) | 0.244 |
| > 65 yr | |||
| Donor sex | |||
| Male | 43 (57.3) | 55 (44) | 0.068 |
| Female | 32 (42.7) | 70 (56) | |
| Donor ICU stay > 6 d | 22 (29.3) | 17 (13.6) | 0.007 |
| Rescue organ | 35 (46.7) | 58 (46.4) | 0.971 |
BC: Biliary complications; ALT init: Alanine aminotransferase initial; WIT/CIT: Warm/cold ischemic time; ICU: Intensive care unit.
Binary logistic regression model
When entering the abovementioned factors in the binary logistic regression model again only length of donor ICU stay had a statistically significant impact on the development of biliary complications in general (P = 0.007, HR = 2.85, 95%CI: 1.33-6.08).
Therapeutic interventions for biliary complications
In Table 6 we summarized the type and frequency of therapeutic interventions in relation to the BCs.
Table 6.
Type and frequency of therapeutic interventions in relation to the biliary complications n (%)
| Percental incidence (Of total n = 200) | Therapy 0 (No consequence) | Therapy 1 (lab control) | Therapy 2 (ERCP/PTCD) | Therapy 3 (OP) | |
| Anastomotic-stenosis grades | 38 (19) | ||||
| 1 | 23 (60.5) | 0 | 21 (91.3) | 2 (8.7) | 0 |
| 2 | 3 (7.9) | 0 | 0 | 3 (100) | 0 |
| 3 | 7 (18.4) | 0 | 2 (28.6) | 5 (71.4) | 0 |
| 4 | 5 (13.2) | 0 | 0 | 5 (100) | 0 |
| Bile leakage | 12 (6) | 1 (8.3) | 2 (16.7) | 3 (25) | 6 (50) |
| Biliary drain complication | 12 (6) | 7 (58.3) | 1 (8.3) | 2 (16.7) | 2 (16.7) |
| Papillary stenosis | 7 (3.5) | 2 (28.57) | 1 (14.29) | 4 (57.14) | 0 |
| ITBL | 6 (3) | 0 | 0 | 6 (100) | 0 |
ITBL: Ischemic type biliary lesion; ERCP/PTCD: Endoscopic retrograde cholangiopancreatography/percutane transhepatic cholangiodrainage; OP: Operation.
Only two patients with anastomotic strictures (grade 1) (8.7%) had to be treated by an interventional procedure. For the others repeated lab control(s) and daily clinical observation were performed. If lab results did not improve, interventional therapy was applied. Patients with complications grade two and higher needed interventions in most cases.
In four patients (57.1%) with papillary stenosis ERCP was also the choice of treatment.
T-tube complications didn’t need any therapy in 58.3% (n = 7), whereas two (16.7%) patients needed ERCP intervention. In two others we had to remove the T-tube surgically.
We had six patients with ITBL. All were treated by ERCP.
In 50% (n = 6) of bile leaks, patients underwent surgical therapy, whereas 25% (n = 3) received ERCP intervention. The remaining 25% resolved spontaneously.
Short and long term outcome
In Table 7 short and long term outcomes are shown. 15 (7.5%) patients who developed cholangitis due to their biliary complication or after interventional therapy anti-infective therapy was also necessary. Six of them developed cholangitis on the basis of anastomotic stenosis, five due to ITBL, three due to papillary stenosis and one patient during manifestation of bile leak.
Table 7.
Short and long term outcome in relation to reconstruction technique, ischemic times and patient groups n (%)
| Cholangitis | ITBL | Rehospitalisation | Re-LT | Death | |
| Rates in total | 15 (7.5 ) | 6 (3 ) | 21 (10.5) | 5 (2.5) | 21 (10.5) |
| Type of reconstruction | |||||
| SS | 14 (93.3) | 6 (100) | 19 (95) | 5 (100) | 1 (4.8) |
| EE | 1 (6.7) | 0 (0) | 2 (5) | 0 (0) | 0 (0) |
| HJ | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Ischemic times | |||||
| CIT > 10 h | 2 (13.3) | 1 (16.6) | 4 (19) | 1 (20) | 1 (4.8) |
| WIT > 45 min | 6 (40) | 1 (16.6) | 9 (42.8) | 3 (60) | 0 (0) |
| Without complications | 0 (0) | 0 (0) | 0 (0) | 1 (20) | 0 (0) |
| Anastomotic stenosis | 6 (40) | 0 (0) | 11 (52.4) | 2 (40) | 1 (4.8) |
| Bile leakage | 1 (6.6) | 0 (0) | 1 (4.8) | 1 (20) | 0 (0) |
| Biliary drain complication | 0 (0) | 0 (0) | 1 (4.8) | 0 (0) | 0 (0) |
| Papillary stenosis | 3 (20) | 0 (0) | 3 (14.3) | 0 (0) | 0 (0) |
| ITBL | 5 (33.4) | 6 (100) | 5 (23.7) | 1 (20) | 0 (0) |
SS: Side-to side; EE: End-to-end; HJ: Hepeaticojejunostomy; CIT: Cold ischemic time; WIT: Warm ischemic time; ITBL: Ischemic type biliary lesion; LT: Liver transplantation.
A patient was found, who developed ITBL as late additional complication. Initially this patient was transplanted because of an alcoholic liver cirrhosis. In the further late patient course (20 mo after LT) he developed an ITBL, leading to a progressive acute liver injury, which was not able to be treated conservative any more. Therefore we performed retransplantation procedure as the last curative possibility. The five other patients listed in Table 7 were diagnosed with ITBL as primary complication before.
Twenty-one (10.5%) patients needed to be rehospitalised because of BCs after LT in total. There were eleven patients with anastomotic stenosis, five with ITBL, three with papillary stenosis and one patient with a bile leak. They all needed intervention or reintervention by ERCP. In one other case, small parts of the T-tube stayed in situ after removal, so that surgical recovery became necessary.
In 5 (2.5%) patients we performed retransplantation procedure. In one case because of an acute liver injury by ITBL as mentioned above. Three patients developed acute liver injury by organ rejection and one patient developed an acute liver injury because of an occlusion of the hepatic artery.
In our series 21 (10.5%) patients died within the follow up period, one of them because of BC. This was a patient with an anastomotic stenosis, who developed a chologenic sepsis after interventional treatment by ERCP with stent implantation followed by recurrent intrahepatic abscesses and death of chologenic sepsis.
DISCUSSION
Biliary complications still belong to the most frequent complications after LT and lead to significant rates of morbidity and mortality[1-5].
The BC incidence in our series was 37.5% (n = 75). Percent of 49.4 of these (n = 37) were only radiological findings not showing any clinical symptoms or elevated lab results. These cases mostly needed lab controls and only in two cases a therapeutic intervention was necessary. This results in an overall clinically relevant incidence of 19% (n = 38 of 200 LT). A number that is comparable to many other series[3,24,25]. As described earlier, most BCs appeared within the first three months.
Overall the incidence of BCs in DD LT is reported to be dependent on a variety of independent factors, such as the type of liver transplant procedure (full size or partial graft), organ preservation, hepatic artery thrombosis, the use of an external or internal biliary drainage, prolonged cold and warm ischemic times, living donor LT, immunological and other specific donor and recipient characteristics[3,22,25-27].
An additional decisive aspect is the type of surgical reconstruction of the biliary system. DD reconstruction and hepaticojejunostomy are standardized techniques which are widely employed, whereas the latter is commonly used in cases of pre-existing biliary disease (e.g., PSC) or if DD reconstruction is not possible[23]. However today there is still no definitive consensus which technique leads to the best patient outcome with less BCs.
Some earlier studies[26,28,29] reported HJ to be accompanied with more frequent complications than DD reconstruction in DDLT. In contrast to these results it was reported, that DD reconstruction in patients undergoing LDLT are associated with a higher risk of BCs. In these cases HJ may be the better choice[30-32].
In our own study, we compared each type of biliary reconstruction technique in relation to the incidence of biliary complications. Within the group of HJ, we didn’t find any anastomotic stricture at all. Compared to the incidence of AS in the other groups, this was statistically significant and contrasts the above mentioned studies[26,28,29]. Concerning all other groups of BCs, the different types of surgical techniques had no significant impact. In comparison to HJ, DD anastomoses are technically simpler and preserve the sphincter Oddi as a natural barrier to bacterial reflux into the biliary tract. Thus it is thought to protect from ascending infections and septic consequences[33]. Furthermore this technique correlates with shorter operation times[26,33]. Another substantial advantage is the possibility to use endoscopic diagnostics and/or interventional therapy, if needed.
Concerning DD anastomoses, Neuhaus et al[34] published already in 1994 the SS reconstruction to be more reliable than other techniques and thus leading to a reduced technical complication rate. Some years later Davidson et al[35] showed in a prospective randomized trial, that there is no difference in relation to the postoperative BCs, so that both techniques EE as well as SS were reported as equally effective. Inserting a T-tube is still a matter of discussion, because most cases of bile leaks are seen at the T-tube insertion site. In addition, removal of the T-tube has been described to lead to further complications[19,35]. On the other hand some authors reported a reduced incidence of anastomotic strictures[36]. In 2006 Weiss et al[21] showed in a large prospective randomized trial that there is a significant increase of complications in patients without T-tube.
According to the recommendations of the Neuhaus group we regularly perform a SS CC with T-tube in our centre. The increased biliary leakage rate reported by others[36,37] was not seen in this series. Overall T-tube complications needing therapeutic interventions occurred only in about 2% of the cases with T-tube.
In our group of patients with EE anastomosis no bile leaks, biliary drain complications, papillary stenoses or appearance of ITBL were detected. However taking into account, the low number of patients (n = 18) in this group we cannot draw any conclusions favouring this procedure over the SS technique.
BCs like bile leaks can be caused by inadequate surgical technique as well as ischemic injury due to arterial perfusion problems, which may be related to the increasing acceptance of so called “marginal donor” organs[38]. Ischemic times (CIT,WIT) may also be influencing factors: Park et al[39] showed in a multivariate analysis, that prolonged CIT is a significant risk factor for BS in patients after LDLT with a DD reconstruction. Kasahara et al[40] on the other hand could not confirm these results. The impact of CIT in DDLT is still discussed controversially. In the early studies of the 1990’s Sanchez-Urdazpal et al[41] and Colonna et al[28] found a significant impact of CIT, whereas Scotté et al[42] could not confirm this. In a more recent study Foley et al[43] found a CIT over 8 h to be the strongest predictor of ischemic cholangiopathy. In contrast, our results show, that CIT as well as WIT were not significantly longer in patients with BCs compared to those without. If we look more closely at patients with anastomotic strictures needing therapeutic interventions (n = 15), only 27% had a CIT over ten hours. Due to the relatively short median CIT of 503 min (mean 493 ± 134) we cannot evaluate the influence of CIT on BCs thoroughly.
An increased ITBL frequency was seen in patients with prolonged CIT[28]. It was suggested that prolonged CIT may injure the microvasculature of the biliary tree and therefore lead to ITBL[25]. In 2010 Heidenhain et al[44] also reported CIT to be a significant risk factor for ITBL. The authors of this paper strongly recommended to keep CIT below ten hours. ITBL was diagnosed only in six cases of our cohort. Among these patients was just one with a CIT over ten hours; a median CIT of 495 min of all ITBL cases was found. Due to the very small number of ITBL cases in our series these results have to be interpreted cautiously.
Donor age was identified as another important factor for development of BCs, in particular AS[45]. Other authors showed no higher rates of AS in elder donors[46], but they found more NAS in patients with donor organs older than 60 years. In our results BC were not statistically more frequent in recipients of organs > 65 years.
Marginal organs are reported to influence BCs[45]. In our data marginal organs in general (rescue allocation) did not, but one extended donor criterion (EDC) (length of ICU stay)[47] did. While the definition of EDC by the German Medical Association implies > 7 ICU days, in our analysis already 6 ICU days showed a significant impact.
Other factors we looked at (donor sex, increased levels of ALT/bilirubin on the first postoperative day) did not show any significant difference concerning the appearance of BCs.
In our study BCs led to higher rehospitalisation rates and consecutively higher costs, but they did not lead to significantly higher rates of retransplantation or death.
Although in our series ischemic times played no explicit role in the development of BCs, other authors showed a significant impact. We recommend to keep ischemic times (CIT, WIT) as low as possible, with special regard to the progress of increasing numbers of marginal donor organs. According to our experiences, performing biliary anastomosis by SS CC with T-tube insertion, is a reliable reconstruction technique and should be applied when technically possible. In contrast to some authors, in our experience, removal of the T-tube can be performed easily without any consequences in general. Removal of the T-tube is performed not until six weeks after LT, so that a newly build tissue tract exists around the tube. Earlier removal or using larger sizes might explain worse experiences. Our study has several limitations. First of all, it is a longitudinal retrospective analysis of single-centre data. Our patient collective of 200 individuals is not very large. However, all surgical procedures were performed by only four surgeons all employing the same technique which makes results more comparable.
In conclusion, technique of biliary reconstruction does not have an impact on the development of biliary complications in our cohort. Neither the increased acceptance of marginal donor grafts in general nor the regular application of T-tubes had a negative significant influence on BC development. However length of donor ICU stay seems to influence the incidence of BCs. The vast majority of BCs can be treated successfully with very few patients requiring revision surgery.
COMMENTS
Background
Biliary complications (BC) represent a significant problem for patients after liver transplantation (LT). Several different factors may impact the occurrence of BC: Graft ischemic time, donor age, donor intensive care unit (ICU) stay, impaired arterial graft perfusion and anastomotic technique are of critical relevance. As different surgical techniques are employed in different centers the data is very heterogeneous and no clear recommendation can be deducted. In this single-center study the authors analyze the occurrence and clinical relevance of BC after LT with special regard to the anastomotic technique.
Research frontiers
No clear gold-standard technique for bile-duct anastomosis after LT exists today. This study aims to clarify the picture.
Innovations and breakthroughs
The authors’ study demonstrates that both end-to-end and side-to-side bile duct anastomoses are of equal quality in the authors’ patient collective. Biliodigestive anastomosis has its place for patients with primary sclerosing cholangitis and can be employed with similar success as direct bile-duct anastomosis. Of all widely accepted factors influencing BC only donor ICU stay > 6 d was relevant in our patient collective.
Applications
The authors’ study demonstrates the patency of different anastomotic techniques for biliary reconstruction in LT. In order to serve each individual patient best different surgical techniques may be considered and employed individually.
Peer-review
The authors of this paper evaluated the relevance and efficacy of different anastomotic techniques for biliary reconstruction after LT. This single-center analysis demonstrates the patency of the different available techniques with comparable results.
Footnotes
Institutional review board statement: This study was reviewed and approved by the Ethics Committee of the Aachen Medical University Hospital.
Informed consent statement: Patients were not required to give informed consent to the study because the analysis used anonymous clinical data that were obtained after each patient agreed to the treatment by written consent. For full disclosure, the details of the study are published on the home page of Aachen Medical University.
Conflict-of-interest statement: We have no financial relationships to disclose.
Data sharing statement: No additional data are available.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: June 24, 2015
First decision: September 17, 2015
Article in press: October 27, 2015
P- Reviewer: Abdel-Wahab M, Chiu KW, Tannuri U, Waisberg J
S- Editor: Qi Y L- Editor: A E- Editor: Li D
References
- 1.Khaderi S, Guiteau J, Cotton RT, O‘Mahony C, Rana A, Goss JA. Role of liver transplantation in the management of hepatoblastoma in the pediatric population. World J Transplant. 2014;4:294–298. doi: 10.5500/wjt.v4.i4.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ostroff JW. Post-transplant biliary problems. Gastrointest Endosc Clin N Am. 2001;11:163–183. [PubMed] [Google Scholar]
- 3.Verdonk RC, Buis CI, Porte RJ, van der Jagt EJ, Limburg AJ, van den Berg AP, Slooff MJ, Peeters PM, de Jong KP, Kleibeuker JH, et al. Anastomotic biliary strictures after liver transplantation: causes and consequences. Liver Transpl. 2006;12:726–735. doi: 10.1002/lt.20714. [DOI] [PubMed] [Google Scholar]
- 4.Boraschi P, Donati F. Complications of orthotopic liver transplantation: imaging findings. Abdom Imaging. 2004;29:189–202. doi: 10.1007/s00261-003-0109-8. [DOI] [PubMed] [Google Scholar]
- 5.Valls C, Alba E, Cruz M, Figueras J, Andía E, Sanchez A, Lladó L, Serrano T. Biliary complications after liver transplantation: diagnosis with MR cholangiopancreatography. AJR Am J Roentgenol. 2005;184:812–820. doi: 10.2214/ajr.184.3.01840812. [DOI] [PubMed] [Google Scholar]
- 6.Palanisamy AP, Taber DJ, Sutter AG, Nadig SN, Dowden JE, McGillicuddy JW, Baliga PK, Chavin KD. Clinical outcomes and costs associated with in-hospital biliary complications after liver transplantation: a cross-sectional analysis. J Gastrointest Surg. 2015;19:282–289. doi: 10.1007/s11605-014-2675-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thuluvath PJ, Pfau PR, Kimmey MB, Ginsberg GG. Biliary complications after liver transplantation: the role of endoscopy. Endoscopy. 2005;37:857–863. doi: 10.1055/s-2005-870192. [DOI] [PubMed] [Google Scholar]
- 8.Park JS, Kim MH, Lee SK, Seo DW, Lee SS, Han J, Min YI, Hwang S, Park KM, Lee YJ, et al. Efficacy of endoscopic and percutaneous treatments for biliary complications after cadaveric and living donor liver transplantation. Gastrointest Endosc. 2003;57:78–85. doi: 10.1067/mge.2003.11. [DOI] [PubMed] [Google Scholar]
- 9.Sawyer RG, Punch JD. Incidence and management of biliary complications after 291 liver transplants following the introduction of transcystic stenting. Transplantation. 1998;66:1201–1207. doi: 10.1097/00007890-199811150-00015. [DOI] [PubMed] [Google Scholar]
- 10.Thethy S, Thomson BNj, Pleass H, Wigmore SJ, Madhavan K, Akyol M, Forsythe JL, James Garden O. Management of biliary tract complications after orthotopic liver transplantation. Clin Transplant. 2004;18:647–653. doi: 10.1111/j.1399-0012.2004.00254.x. [DOI] [PubMed] [Google Scholar]
- 11.Mahajani RV, Cotler SJ, Uzer MF. Efficacy of endoscopic management of anastomotic biliary strictures after hepatic transplantation. Endoscopy. 2000;32:943–949. doi: 10.1055/s-2000-9619. [DOI] [PubMed] [Google Scholar]
- 12.Akamatsu N, Sugawara Y, Hashimoto D. Biliary reconstruction, its complications and management of biliary complications after adult liver transplantation: a systematic review of the incidence, risk factors and outcome. Transpl Int. 2011;24:379–392. doi: 10.1111/j.1432-2277.2010.01202.x. [DOI] [PubMed] [Google Scholar]
- 13.Morelli J, Mulcahy HE, Willner IR, Cunningham JT, Draganov P. Long-term outcomes for patients with post-liver transplant anastomotic biliary strictures treated by endoscopic stent placement. Gastrointest Endosc. 2003;58:374–379. doi: 10.1067/s0016-5107(03)00011-7. [DOI] [PubMed] [Google Scholar]
- 14.Holt AP, Thorburn D, Mirza D, Gunson B, Wong T, Haydon G. A prospective study of standardized nonsurgical therapy in the management of biliary anastomotic strictures complicating liver transplantation. Transplantation. 2007;84:857–863. doi: 10.1097/01.tp.0000282805.33658.ce. [DOI] [PubMed] [Google Scholar]
- 15.Verdonk RC, Buis CI, Porte RJ, Haagsma EB. Biliary complications after liver transplantation: a review. Scand J Gastroenterol Suppl. 2006;(243):89–101. doi: 10.1080/00365520600664375. [DOI] [PubMed] [Google Scholar]
- 16.Buis CI, Verdonk RC, Van der Jagt EJ, van der Hilst CS, Slooff MJ, Haagsma EB, Porte RJ. Nonanastomotic biliary strictures after liver transplantation, part 1: Radiological features and risk factors for early vs. late presentation. Liver Transpl. 2007;13:708–718. doi: 10.1002/lt.21166. [DOI] [PubMed] [Google Scholar]
- 17.Howell JA, Gow PJ, Angus PW, Jones RM, Wang BZ, Bailey M, Fink MA. Early-onset versus late-onset nonanastomotic biliary strictures post liver transplantation: risk factors reflect different pathogenesis. Transpl Int. 2012;25:765–775. doi: 10.1111/j.1432-2277.2012.01501.x. [DOI] [PubMed] [Google Scholar]
- 18.Pfau PR, Kochman ML, Lewis JD, Long WB, Lucey MR, Olthoff K, Shaked A, Ginsberg GG. Endoscopic management of postoperative biliary complications in orthotopic liver transplantation. Gastrointest Endosc. 2000;52:55–63. doi: 10.1067/mge.2000.106687. [DOI] [PubMed] [Google Scholar]
- 19.Ostroff JW, Roberts JP, Gordon RL, Ring EJ, Ascher NL. The management of T tube leaks in orthotopic liver transplant recipients with endoscopically placed nasobiliary catheters. Transplantation. 1990;49:922–924. doi: 10.1097/00007890-199005000-00018. [DOI] [PubMed] [Google Scholar]
- 20.Shuhart MC, Kowdley KV, McVicar JP, Rohrmann CA, McDonald MF, Wadland DW, Emerson SS, Carithers RL, Kimmey MB. Predictors of bile leaks after T-tube removal in orthotopic liver transplant recipients. Liver Transpl Surg. 1998;4:62–70. doi: 10.1002/lt.500040109. [DOI] [PubMed] [Google Scholar]
- 21.Weiss S, Schmidt SC, Ulrich F, Pascher A, Schumacher G, Stockmann M, Puhl G, Guckelberger O, Neumann UP, Pratschke J, et al. Biliary reconstruction using a side-to-side choledochocholedochostomy with or without T-tube in deceased donor liver transplantation: a prospective randomized trial. Ann Surg. 2009;250:766–771. doi: 10.1097/SLA.0b013e3181bd920a. [DOI] [PubMed] [Google Scholar]
- 22.Sharma S, Gurakar A, Jabbour N. Biliary strictures following liver transplantation: past, present and preventive strategies. Liver Transpl. 2008;14:759–769. doi: 10.1002/lt.21509. [DOI] [PubMed] [Google Scholar]
- 23.Welsh FK, Wigmore SJ. Roux-en-Y Choledochojejunostomy is the method of choice for biliary reconstruction in liver transplantation for primary sclerosing cholangitis. Transplantation. 2004;77:602–604. doi: 10.1097/01.tp.0000113807.74942.d2. [DOI] [PubMed] [Google Scholar]
- 24.Karimian N, Westerkamp AC, Porte RJ. Biliary complications after orthotopic liver transplantation. Curr Opin Organ Transplant. 2014;19:209–216. doi: 10.1097/MOT.0000000000000082. [DOI] [PubMed] [Google Scholar]
- 25.Pascher A, Neuhaus P: Biliary complications after deceased-donor orthotopic liver transplantation. J Hepatobiliary Pancreat Surg. 2006;13:487–496. doi: 10.1007/s00534-005-1083-z. [DOI] [PubMed] [Google Scholar]
- 26.Greif F, Bronsther OL, Van Thiel DH, Casavilla A, Iwatsuki S, Tzakis A, Todo S, Fung JJ, Starzl TE. The incidence, timing, and management of biliary tract complications after orthotopic liver transplantation. Ann Surg. 1994;219:40–45. doi: 10.1097/00000658-199401000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seehofer D, Eurich D, Veltzke-Schlieker W, Neuhaus P. Biliary complications after liver transplantation: old problems and new challenges. Am J Transplant. 2013;13:253–265. doi: 10.1111/ajt.12034. [DOI] [PubMed] [Google Scholar]
- 28.Colonna JO, Shaked A, Gomes AS, Colquhoun SD, Jurim O, McDiarmid SV, Millis JM, Goldstein LI, Busuttil RW. Biliary strictures complicating liver transplantation. Incidence, pathogenesis, management, and outcome. Ann Surg. 1992;216:344–350; discussion 350-352. doi: 10.1097/00000658-199209000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O‘Connor TP, Lewis WD, Jenkins RL. Biliary tract complications after liver transplantation. Arch Surg. 1995;130:312–317. doi: 10.1001/archsurg.1995.01430030082017. [DOI] [PubMed] [Google Scholar]
- 30.Hwang S, Lee SG, Sung KB, Park KM, Kim KH, Ahn CS, Lee YJ, Lee SK, Hwang GS, Moon DB, et al. Long-term incidence, risk factors, and management of biliary complications after adult living donor liver transplantation. Liver Transpl. 2006;12:831–838. doi: 10.1002/lt.20693. [DOI] [PubMed] [Google Scholar]
- 31.Kyoden Y, Tamura S, Sugawara Y, Matsui Y, Togashi J, Kaneko J, Kokudo N, Makuuchi M. Incidence and management of biliary complications after adult-to-adult living donor liver transplantation. Clin Transplant. 2010;24:535–542. doi: 10.1111/j.1399-0012.2009.01124.x. [DOI] [PubMed] [Google Scholar]
- 32.Seo JK, Ryu JK, Lee SH, Park JK, Yang KY, Kim YT, Yoon YB, Lee HW, Yi NJ, Suh KS. Endoscopic treatment for biliary stricture after adult living donor liver transplantation. Liver Transpl. 2009;15:369–380. doi: 10.1002/lt.21700. [DOI] [PubMed] [Google Scholar]
- 33.Sung JY, Costerton JW, Shaffer EA. Defense system in the biliary tract against bacterial infection. Dig Dis Sci. 1992;37:689–696. doi: 10.1007/BF01296423. [DOI] [PubMed] [Google Scholar]
- 34.Neuhaus P, Blumhardt G, Bechstein WO, Steffen R, Platz KP, Keck H. Technique and results of biliary reconstruction using side-to-side choledochocholedochostomy in 300 orthotopic liver transplants. Ann Surg. 1994;219:426–434. doi: 10.1097/00000658-199404000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Davidson BR, Rai R, Kurzawinski TR, Selves L, Farouk M, Dooley JS, Burroughs AK, Rolles K. Prospective randomized trial of end-to-end versus side-to-side biliary reconstruction after orthotopic liver transplantation. Br J Surg. 1999;86:447–452. doi: 10.1046/j.1365-2168.1999.01073.x. [DOI] [PubMed] [Google Scholar]
- 36.Scatton O, Meunier B, Cherqui D, Boillot O, Sauvanet A, Boudjema K, Launois B, Fagniez PL, Belghiti J, Wolff P, et al. Randomized trial of choledochocholedochostomy with or without a T tube in orthotopic liver transplantation. Ann Surg. 2001;233:432–437. doi: 10.1097/00000658-200103000-00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vougas V, Rela M, Gane E, Muiesan P, Melendez HV, Williams R, Heaton ND. A prospective randomised trial of bile duct reconstruction at liver transplantation: T tube or no T tube? Transpl Int. 1996;9:392–395. doi: 10.1007/BF00335701. [DOI] [PubMed] [Google Scholar]
- 38.Wojcicki M, Lubikowski J, Klek R, Post M, Jarosz K, Białek A, Wunch M, Czuprynska M. Reduction of biliary complication rate using continuous suture and no biliary drainage for duct-to-duct anastomosis in whole-organ liver transplantation. Transplant Proc. 2009;41:3126–3130. doi: 10.1016/j.transproceed.2009.07.091. [DOI] [PubMed] [Google Scholar]
- 39.Park JB, Kwon CH, Choi GS, Chun JM, Jung GO, Kim SJ, Joh JW, Lee SK. Prolonged cold ischemic time is a risk factor for biliary strictures in duct-to-duct biliary reconstruction in living donor liver transplantation. Transplantation. 2008;86:1536–1542. doi: 10.1097/TP.0b013e31818b2316. [DOI] [PubMed] [Google Scholar]
- 40.Kasahara M, Egawa H, Takada Y, Oike F, Sakamoto S, Kiuchi T, Yazumi S, Shibata T, Tanaka K. Biliary reconstruction in right lobe living-donor liver transplantation: Comparison of different techniques in 321 recipients. Ann Surg. 2006;243:559–566. doi: 10.1097/01.sla.0000206419.65678.2e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sanchez-Urdazpal L, Gores GJ, Ward EM, Maus TP, Wahlstrom HE, Moore SB, Wiesner RH, Krom RA. Ischemic-type biliary complications after orthotopic liver transplantation. Hepatology. 1992;16:49–53. doi: 10.1002/hep.1840160110. [DOI] [PubMed] [Google Scholar]
- 42.Scotté M, Dousset B, Calmus Y, Conti F, Houssin D, Chapuis Y. The influence of cold ischemia time on biliary complications following liver transplantation. J Hepatol. 1994;21:340–346. doi: 10.1016/s0168-8278(05)80311-3. [DOI] [PubMed] [Google Scholar]
- 43.Foley DP, Fernandez LA, Leverson G, Anderson M, Mezrich J, Sollinger HW, D‘Alessandro A. Biliary complications after liver transplantation from donation after cardiac death donors: an analysis of risk factors and long-term outcomes from a single center. Ann Surg. 2011;253:817–825. doi: 10.1097/SLA.0b013e3182104784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Heidenhain C, Pratschke J, Puhl G, Neumann U, Pascher A, Veltzke-Schlieker W, Neuhaus P. Incidence of and risk factors for ischemic-type biliary lesions following orthotopic liver transplantation. Transpl Int. 2010;23:14–22. doi: 10.1111/j.1432-2277.2009.00947.x. [DOI] [PubMed] [Google Scholar]
- 45.Sundaram V, Jones DT, Shah NH, de Vera ME, Fontes P, Marsh JW, Humar A, Ahmad J. Posttransplant biliary complications in the pre- and post-model for end-stage liver disease era. Liver Transpl. 2011;17:428–435. doi: 10.1002/lt.22251. [DOI] [PubMed] [Google Scholar]
- 46.Serrano MT, Garcia-Gil A, Arenas J, Ber Y, Cortes L, Valiente C, Araiz JJ. Outcome of liver transplantation using donors older than 60 years of age. Clin Transplant. 2010;24:543–549 [. doi: 10.1111/j.1399-0012.2009.01135.x. [DOI] [PubMed] [Google Scholar]
- 47.Association GM. Guidelines for organ transplantation §16 TPG. Dtsch Artebl. 2004;5:246–247. [Google Scholar]