Abstract
Adapting to an over-demanding stressful urban environment may exhaust the psychophysiological resources to cope with these demands, and lead to sympathetic nervous system dysfunction. The evidence that an urban-dwelling lifestyle may be detrimental to the cardiometabolic health of Africans motivated the design of the Sympathetic activity and Ambulatory Blood Pressure in African Prospective cohort study. We aimed to determine neural mechanistic pathways involved in emotional distress and vascular remodelling. The baseline sample included 409 teachers representing a bi-ethnic sex cohort from South Africa. The study was conducted in 2008–09 and repeated after 3-year follow-up in 2011–12, with an 87.8% successful follow-up rate. Seasonal changes were avoided and extensive clinical assessments were performed in a well-controlled setting. Data collection included sociodemographics, lifestyle habits, psychosocial battery and genetic analysis, mental stress responses mimicking daily life stress (blood pressure and haemostatic, cardiometabolic, endothelial and stress hormones). Target organ damage was assessed in the brain, heart, kidney, blood vessels and retina. A unique highly phenotyped cohort is presented that can address the role of a hyperactive sympathetic nervous system and neural response pathways contributing to the burden of cardiometabolic diseases in Africans.
Key Messages.
Cardiometabolic vulnerability was evident in an urban African male cohort.
The brain-heart link revealed higher sympathetic activity via depressed heart variability, α-adrenergic vascular, silent ischaemia and attenuated neuroendocrine and psychological distress responses. These findings were associated with hypertrophic remodelling and compensatory raised ambulatory blood pressure.
Hypervigilant defensive coping facilitated disturbed cardiometabolic responses: self-reported behavioural ‘in-control’ defensive coping was coupled with physiological ‘loss-of-control’ defensive coping responses. This demonstrates dissociation between defensive coping behaviour and physiology, which may mask control and induce neural fatigue or ‘burn-out’.
Why was the cohort set up?
Cross-sectional surveys over more than 20 years have demonstrated hyperactive sympathetic nervous system (SNS) responses in approximately 9000 Blacks (Africans) from the North-West Province in South Africa.1–4 SNS responses and prevalence of lifestyle-related diseases were apparent in urban-dwelling Africans, which underscored the urban environment as a potent chronic psychosocial stressor.1–4 Other studies support the role of SNS hyperactivity or early stages of neurogenic hypertension associated with cardiometabolic risk markers such as disturbed vascular blood pressure responses,2 stress hormones,5 inflammation,6 fibrinolysis,7 structural wall changes in the left ventricle8 and sympathovagal disturbance.9 Thus the process of chronic exposure to psychosocial stress will result in high allostatic load or overload, which may be detrimental to cardiometabolic health.10 It is possible that prolonged, uncontrollable psychosocial stress impairs stress appraisal or coping ability at both physiological and psychological levels, inducing neural fatigue.2,3 Despite improved medical treatment regimens, the emerging burden of cardiometabolic disease among urban Black Africans urgently needed an innovative approach. To date, experimental trials of hypertension treatment or prospective population studies have not attempted to explain the role of hyperactive SNS or neural response pathways (brain) contributing to the cardiometabolic (heart) disease burden in Africans. Therefore, an innovative brain-heart link approach motivated the design of the international Metabolic Syndrome Institute Award-winning SABPA Project. Our objectives were to conduct the first psychophysiological prospective cohort study in sub-Saharan Africa, addressing (i) the brain-heart link and (ii) neural response pathways to describe plausible mechanisms for cardiometabolic morbidity and mortality in a bi-ethnic cohort including both sexes.
Who is in the cohort?
All teachers (N = 2170), enrolled in the 43 schools of the Dr Kenneth Kaunda Education District (Klerksdorp and Potchefstroom), North-West Province South Africa, were invited to participate (Figures 1–2). To control for seasonal variation of the pro-coagulation markers, only approximately 200 participants could participate annually (Figure 3).11 Power analyses were therefore performed for the SABPA study cohort. Using previous studies, ambulatory autonomic dysfunction12 and cortisol data13,14 were used to obtain relevant effect sizes based on differences in biological profiles and genotyping hypothalamic-pituitary-adrenal (HPA) axis variation. Resulting sample sizes of 50–416 would enable explanation of biological differences and detection of single nucleotide polymorphisms (SNPs) with a statistical power of 0.8, and level of significance of 0.05.12–14
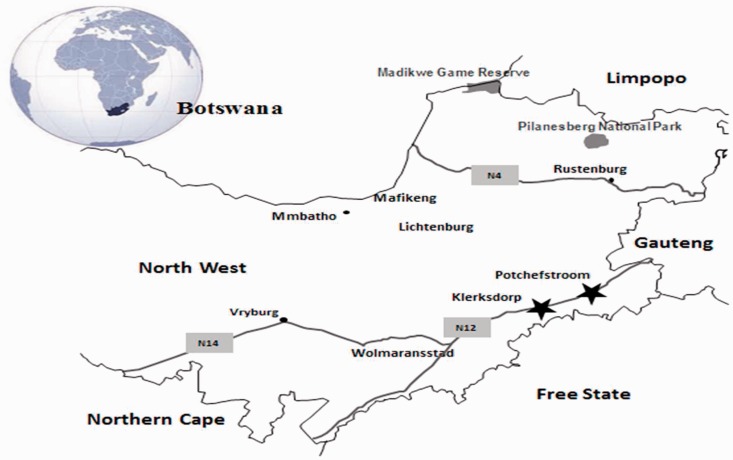
Figure 1.
Geographical location of Klerksdorp and Potchefstroom in the North-West Province, South Africa.
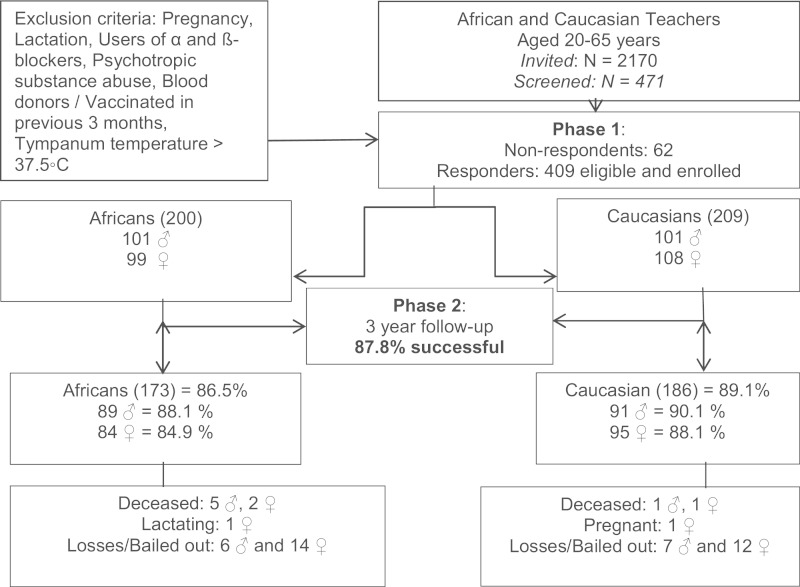
Figure 2.
The Sympathetic activity and Ambulatory Blood pressure in Africans (SABPA) prospective cohort study population.
Figure 3.
Mean temperature over the 4-month data collection periods of the SABPA Prospective cohort study during late summer-late autumn11.
The target population included urban-dwelling well-educated Black (African) and White African (Caucasian) male and female teachers, ensuring participants with similar socioeconomic standing (Figure 2). All volunteering teachers had medical aid benefits and were screened to meet study eligibility criteria during the recruitment phase (Figure 2). Those complying formed the respondent group of 409 (Figure 2), and those not complying formed the non-respondent group (N = 62) (Table 1). The Black teachers preferred to be informed and recruited in separate sex groups and the protocol, especially the amount of blood drawn and hair sampling, was not well received. Time constraints were the main obstacle for participation in the Caucasian teachers’ cohort and mixed-sex informed recruitment sessions were not a problem. Data are currently available for 409 teachers of Phase I from which 359 were followed up in Phase II.
Table 1.
Comparing respondents and non-respondents in terms of basic variables
| Variables | Respondents (N = 409) | Non-respondents (N = 62) | Difference (95% CI) | P-value (t-/X2 tests) |
|---|---|---|---|---|
| Socio-demographic profile at baseline | ||||
| Age, years | 44.70 ± 9.6 | 46.39 9.2 | −1.69 (−4.25–0.87) | 0.195 |
| Sex, men, n (%) | 202 (49.4) | 10 (16.1) | 33.3 (20.2–46.3) | <0.0001 |
| Africans, n (%) | 200 (48.9) | 15 (24.2) | 24.7 (11.5–37.9) | 0.0003 |
| Teachers, grades 1–7, n (%) | 181 (44.3) | 39 (62.9) | 18.6 (5.3–31.9) | 0.006 |
| Self-reported anthropometric measurements | ||||
| Body mass index (kg/m2)a | 28.85 ± 6.6 | 30.26 ± 5.0 | −1.41 (−3.13–0.31) | 0.108 |
| Self-reported comorbidities at baseline | ||||
| Cardiovascular disease, n (%) | 44 (10.8) | 21 (33.9) | 23.1 (14.1–32.1) | <0.0001 |
| Hypertension drugs, n (%) | 96 (23.5) | 27 (43.6) | 20.1 (8.4–31.7) | 0.0008 |
| Diagnosed diabetics, n (%) | 12 (2.9) | 8 (12.9) | 10.0 (4.6–15.3) | 0.0003 |
| Epilepsy, n (%) | 3 (0.73) | 6 (9.68) | 9.0 (5.4–12.5) | <0.0001 |
| Antidepressant drugs, n (%) | 4 (1.0) | 33 (53.2) | 52.2 (46.8–57.7) | <0.0001 |
| Blood donor, n (%) | 0 (0.0) | 6 (9.7) | 9.7 (6.8–12.6) | <0.0001 |
Values are expressed as arithmetic mean or number of participants (%). Differences are expressed as absolute values (95% CI).
The SABPA study abode by the institutional guidelines and terms of the Declaration of Helsinki (revised 2004) and was approved by the ethics review board of the North-West University, Potchefstroom Campus (NWU-0003607S6). Consent and cooperation agreement were obtained from the Department of Education North-West, South African Democratic Teacher Unions and Headmasters of Schools. The nature, benefits and risks of the study were explained in detail in English and native language to the involved parties and teachers, and their written informed consent was obtained before participation.
Recruitment was systematically done over a 3-month period prior to clinical assessments during Phases 1 and 2. A registered nurse and a trained African fieldworker provided volunteers with a detailed description of planned measurements, the protocol and expected outcomes with an opportunity to ask questions. Participants’ medical history and treatment information were screened for eligibility (21.71% of 2170 invited teachers) and, on approval, for inclusion in the main study. This was followed by the signing of informed consent forms by all eligible participants. Clinical assessments were conducted for each participant over a 2-day period during late summer–late autumn 2008 and 2009, and 409 participants completed Phase I (Figure 2). Table 1 compares respondents and non-respondents in terms of basic variables. Men and Africans were under-represented in the non-respondent group. Approximately 19% of all invited teachers (N = 2170), including 44% of all primary school teachers (Grades 1 to 7) participated in the SABPA study (Figure 2, Table 1). Non-respondents used more medications and demonstrated more cardiometabolic comorbidities. The differences almost throughout could be attributed to the tightly controlled lower comorbidities required for the respondent group.
How often have they been followed up?
Phase 1 assessment of SABPA was undertaken over a 1-year period in 2008–09. Follow-up (Phase 2) data collection was completed after 3 years in 2011–12. Contact between baseline and 3-year follow-up was sustained via mobile cellular messaging system and feedback workshops to explain results and referrals. The participants were encouraged to follow medical prescribed treatment regimens resulting from the study’s clinical assessments. Three months prior to 3 years after Phase 1, all respondents who completed Phase I were invited to take part in the follow-up (Figure 1). The follow-up participation rate was high (87.8%). The non-respondents (N = 62) were encouraged to enter the service delivery programme of the Hypertension Research and Training Clinic (on campus) for ambulatory blood pressure screening. Respondents are registered teachers, allowing a trained employee and the principal investigator to keep track of the mortality rates of the respondent group only, in collaboration with the North-West Departments of Education and Internal Affairs. A third follow-up has not been finalized.
What was measured?
The data were collected in a well-controlled overnight facility at the North-West University in order to habituate participants to their environment, to avoid the white-coat effect,15 while maintaining high data quality. Well-controlled conditions were maintained in assessing optimal SNS responses during exposure to acute mental laboratory stressors.16 Individualized health profile reports and referrals were received by each respondent within 1 week after data collection. Despite the intense clinical assessment programme, feedback reports indicated that participants were comfortable with the experimental setting and 97.8% expressed their interest for future participation.
Seasonal variations were avoided and gold-standard methods were applied at both phases, i.e. validated questionnaires, measurements and calibrated apparatus (Figure 3; Table 2). Repeat measurements were collected at both phases 1 and 2 of the brain-heart link measures [HPA and sympathy-adrenal-medullary axis (SAM), renin-angiotensin-aldosterone system (RAAS), ambulatory blood pressure and electrocardiogram (ECG), and objectively assessed lifestyle (Figure 4), neurotrophins, renal function, metabolism, pro-inflammatory, pro-thrombotic, functional and structural endothelium]. The psychosocial battery was completed under supervision of bi-ethnic clinical psychologists. Psychophysiological stress tasks, namely the Cold Pressor and the Stroop Word-Colour Conflict (Stroop) tests16 were administered at phase I and were applied for 1 min in a counterbalanced design. For blood sampling, a sterile winged infusion set was left in situ with a heparin block to prevent clotting (0.5 ml of heparin sodium-fresenius 5000 IU/ml in 50 ml normal saline solution; Fresenius Kabi, Port Elizabeth, South Africa). The infusion set was thoroughly flushed with 2–3 ml saline and the first 2 ml of blood discarded, before sampling was done for coagulation measures. For each task, beat-to-beat blood pressure and 12-lead ECG were assessed throughout stress testing followed by a 10-min recovery period. Blood (total 150 ml) and saliva sampling (total 6 ml) was done at rest and at 10 and 30 min post-task, respectively. Participants received a monetary incentive according to performance during completion of the Stroop task as motivation to excel, and perception of stressors was scored. A medical doctor was available for adverse events. Additional measurements at phase 2 included 24-h diet, 24-h urine, 7-day objective physical activity status, differentiated blood cell count, genetic damage, microvascular (retinal) endothelial function under mydriatic conditions, and chronic stress (hair samples) (Table 2).
Table 2.
Summary of brain-heart clinical assessments and apparatus
| Assessments | 2008–09 | 2011–12 |
|---|---|---|
| Medical history & related questionnaires: | √ | √ |
| Duration of stay, education, marital status, alive family members, health (cardiometabolic, inflammation, depression, renal, arthritis, cancer, reproduction), sleep apnoea, ambulatory & dietary diary, mental stress perception | ||
| Feedback of results (incl. referrals and incident findings): | √ | √ |
| Individual & group | ||
| Chronic medications | ||
| Nervous system, kidney, hypertension, arrhythmia, diabetes, coagulants, hormones, inflammation, analgesics, depression/anxiety, proton pump, epilepsy, supplements | √ | √ |
| Lifestyle | ||
| Objective & self-reported smoking & alcohol habits | √ | √ |
| Diet, standardized dinner & breakfast | √ | √ 24-h |
| Anthropometry (height, body mass, waist and neck circumferences) | √ | √ |
| Number of times measured (Invicta Stadiometer IP 1465, Precision Health Scale, A & D Company Holtain 7-mm wide metal tape) | 3 | 3 |
| Objective total energy expenditure (Actical® Mini Mitter, Bend OR; 7-days Actiheart, CamNtech Ltd) | √ | √ 7-day |
| Changes in lifestyle habits over time | √ | |
| Psychosocial battery | ||
| Measures with known heritability: life orientation, personality | √ | X |
| Predictors of development/worsening of hypertension: coping, depression, cognitive distress | √ | √ |
| Measures which could moderate the effects of environment: fortitude, mental health, self-regulation, job stress | √ | √ |
| Cardiovascular assessment | ||
| Resting BP & 12-lead ECG (Riester CE 0124® &1.3M ™ Littman® II S.E. Stethoscope 2205; Finometer, Finapres Medical Systems®; NORAV PC-ECG 1200®) | a√ | √ |
| 24-h ambulatory BP & -ECG (Cardiotens® & Cardiovisions 1.19®, Meditech) | √ | √ |
| Biochemical assessments (trained technicians; accredited laboratories) | ||
| Bodily fluids sampling times (urine, blood, saliva) | √ | √ |
| Serum brain-derived neurotrophic factor | √ | √ |
| Serum cotinine (smoking) and liver enzymes (alcohol misuse) | √ | √ |
| Differentiated blood count | Χ | √ |
| Serum electrolytes | √ | √ |
| Serum oxidative stress | a√ | √ |
| Citrate haemostasis | a√ | √ |
| Plasma inflammatory profile, | √ | √ |
| Plasma essential amino acids | √ | √ |
| Plasma renin-angiotensin-aldosterone system, endothelial function (L-arginine-NO synthase) | a√ | √ |
| Urine/blood/saliva HPA & SAM axis hormones, serotonin | a√ | √ hair |
| Whole blood HIV status (PMC Medical, Daman, India), Pareekshak test (BHAT Bio-Tech, Bangalore, India) | √ | √ |
| Retinal vessel analysis | X | √ |
| Static & dynamic measures (incl. dynamic saliva stress hormone responses) (DVA PLUS 12100003®, Imedos) | ||
| Intraocular eye pressure | X | √ |
| (TONO-PEN-AVIA® Applanation Tonometer CE 0120, Reichert) | ||
| Ultrasound carotid intima-media thickness (CIMT) | b√ | √ |
| (Sonosite Micromaxx®, SonoSite Inc., Bothell, WA) | ||
| DNA genome (PCR based) | ||
| Nuclear & mitochondrial genome sequencing | √ | |
| SNP analyses, epigenetics | √ | √ |
| Telomere length | √ | |
| Metabolism | a√ | √ |
| Serum lipogram & insulin, NaF glucose, urine metabonomics | ||
| Renal function | √ | √ 24-h |
| Serum creatinine & 8-h urine |
X, no measures.
aIncluding sympathetic nervous system (SNS) responses (1-min exposure to mental stress (Cold Pressor & Stroop Colour-Word-Conflict tests).
bCIMT, plaque score and stenosis at optimal angles obtained from baseline.17
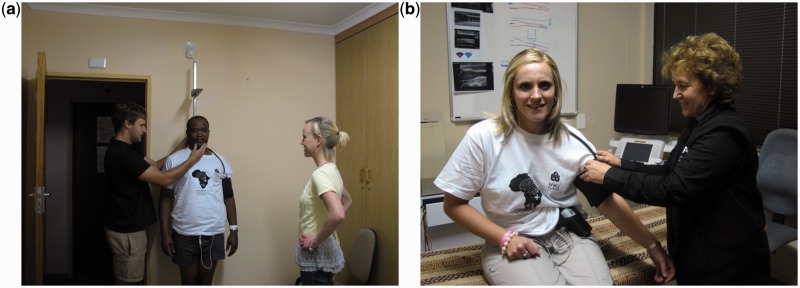
Figure 4.
Obtaining anthropometric (4a) and 24h ambulatory blood pressure and 24h ECG (including heart rate variability) measures (4b) during the SABPA Prospective Cohort study data collection phases.
What has been found?
A complete list of collaborators and publications from the SABPA Study can be found at http://stressed-project.co.za/data-dissemination/.
The overarching aim of the study was to assess the interaction between brain and cardiometabolic responses (brain-heart link) and the neural response pathways in a bi-ethnic cohort including both sexes in order to provide estimates of the proportion of individuals at high risk of future vascular events. We have demonstrated that objective lifestyle risk factors, such as alcohol misuse, mostly explained differences in blood pressure, the metabolic syndrome and sub-clinical vascular disease between Black and White South Africans.18–20 Among Black Africans we observed disturbed sympathovagal balance suggestive of sympathetic activity modulation, augmented α-adrenergic vascular, pro-coagulant, attenuated nitric oxide metabolite, stress hormone and emotional distress responses.20–29 These factors potentiate adrenergic drive and reinforce metabolic overdrive and structural alterations.20–29 Diminished ß-adrenergic responsiveness was previously suspected in urban Africans2 and is again demonstrated by the increased 24-h BP, augmented α-adrenergic responses and associated vascular hypertrophy in the SABPA cohort.20,24–27 Moreover, our findings complement a notion of autonomic dysfunction via depressed heart rate variability and early structural vascular changes in African men, mostly facilitated by hypervigilant defensive coping.20–22,27 These structural vascular changes could counteract sympathetic vasoconstriction and contributed to their stress-induced ischaemia.20 This will thus impair neuronal re-uptake of norepinephrine, thereby potentiating sympathetic signalling. Indeed, reduced perfusion of the heart was observed with a higher prevalence of silent ischaemic events in hypervigilant defensive coping African men, especially if low testosterone levels prevail, i.e. 9.5 events [95% confidence interval (CI): 5.1-14.0] vs 1.5 (0.0-5.3) when compared with Caucasian counterparts.20–21,27–28
Overall, cardiometabolic vulnerability was more evident in African men compared with African women and Caucasian men and women,18–31 particularly when utilizing defensive coping.20–22,27 Behavioural and physiological defensive coping relates to active problem-solving and primitive ‘fight’, in-control, positive affect33 and eliciting ß-adrenergic responses,32,33 whereas emotional avoidance coping relates to defeat, ‘flight’ or loss-of-control responses, which have been associated with poorer well-being and an α-adrenergic response profile.32,33 However, our findings contradict this notion as African men reported behavioural defensive coping ‘in-control’ responses coupled with physiological emotional avoidance ‘loss-of-control’ responses.20–22,27 Indeed, they revealed more metabolic syndrome markers, attenuated stress hormone levels and autonomic dysfunction.20–22,27 An apparent dissociation evidently occurs between behavioural and physiological defensive coping responses suggesting ‘loss-of-physiological control’. It could further imply that an overly taxing situation or challenging environment, where chronic stress is experienced, may mask control. Subsequent exhaustion of ‘physiological’ resources may occur when control cannot be exerted.20–22,27
Another urgent matter, at the heart of the rising metabolic syndrome epidemic, was the lack of ethnic-specific cut-points for central obesity in prospective studies. We addressed this crucial matter and an ethnic-specific waist circumference (WC) cut-point model for Africans was proposed.19,30 The model was validated by utilizing diagnostic tests and non-linear analyses.19 Furthermore, supporting the brain-heart link, we revealed that the validated ethnic-specific WC cut-point model (African men, ≥90 cm; -women, ≥98 cm) was associated with cognitive emotional distress and sub-clinical atherosclerosis.30
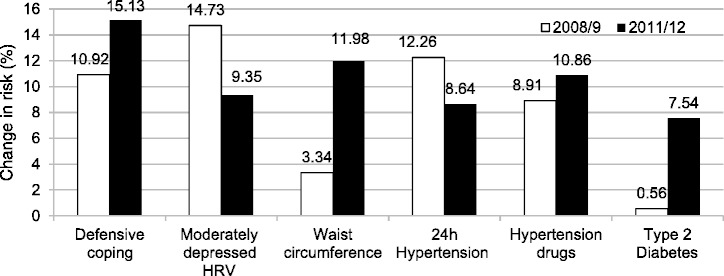
Preliminary causal analyses are progressing and thus far receiver operating characteristic (ROC) WC cut-point models strongly support the use of only WC and blood pressure as predictors of the metabolic syndrome.30–31 McNemar's case-control test was used to calculate change in risk (unpublished, Figure 5). We demonstrated no change in risk for behavioural defensive coping responses {4.21% change, P = 0.147 [odds ratio (OR) 1.39, 95% CI: 0.90-2.15]}. Pertaining to physiological responses, we observed a decreased trend for autonomic dysfunction [−5.38%, P = 0.051 (OR 0.64, 95% CI: 0.40-1.00)], but no change in risk for a hypertensive state [3.62%, P = 0.313 (OR 0.71, 95% CI: 0.43-1.14)] or usage of hypertension drugs [1.95%, P = 0.313 (OR 1.22, 95% CI: 0.74-2.01)]. Increased risk was however revealed for waist circumference [8.64%, P < 0.0001 (OR 3.58, 95% CI: 1.86-7.47)] and type 2 diabetes (6.98%, P < 0.0001) (OR 13.5, 95% CI: 3.4-117.1)]. Findings underscore chronic behavioural defensive ‘in-control’ responses, which were accompanied by physiological pathological changes, possibly indicating ‘loss-of-control’ responses. The ongoing challenge will be to describe neural response pathway mechanisms for cardiometabolic morbidity and mortality from a dissociative stress perspective.
Figure 5.
Cardiometabolic risk markers presenting change in risk over 3 years in the SABPA Prospective cohort. Heart rate variability (HRV) suggests increased autonomic dysfunction (standard deviation R-R interval, 50-100 ms); Waist circumference, Joint Interim Statement34.
What are the main strengths and weaknesses of the study?
Main weaknesses include the relatively small sample size, which was restricted in order to control for seasonal variation. Additionally, the requirement for an overnight stay and 2 days of testing likely may have had a considerable restrictive effect on the initial response rate. Potential limitations are biases of initial non-response or attrition over time. Key strengths are the generation of a unique highly phenotyped cohort in a well-controlled research setting. A complex set of gold-standard measures were obtained in the first psychophysiological prospective cohort study in sub-Saharan Africa. The focus in this study is on SNS activity contributing to cardiometabolic disease and emotional distress. Current findings address the original SABPA design and problem statement and prospective findings may confirm the American Heart Association 2014 statement35 that depression needs to be considered as a risk factor in preventive cardiology.
Storage and information about the data set? Where can I get hold of the data or find out more?
The cohort data and samples are embedded in a single large regional research centre at the study research institute of the Hypertension in Africa Research Team, North-West University (Potchefstroom Campus), South Africa. Data are password protected and electronic participant records are stored for a maximum of 20 years in the secured facility. The electronic records have been flagged to facilitate ease of contact and follow-up. Therefore, in addition to the measures that were undertaken, it will be possible to access measurements undertaken at both phases 1 and 2. To ensure maximum data dissemination, all national and international expert collaborators signed a contract (memorandum of understanding) for sharing of data and author input. Participant confidentiality was maintained under all circumstances. Potential collaborators are invited to contact the corresponding author and principal investigator at [leone.malan@nwu.ac.za]. Any requests to use the data will be reviewed by HART.
Funding
This work was financially supported by: the Metabolic Syndrome Institute France; and the North-West University, Medical Research Council, National Research Foundation, PA & Alize Malan Trust, North-West Department of Education and Roche Diagnostics, South Africa. The funding organizations played no role in the design or conduct of the study; collection, management, analysis and interpretation of the data; or preparation, review or approval of the manuscript.
Acknowledgements
The SABPA study would not have been possible without the volunteering participant sample, the dedicated input of the Hypertension in Africa Research Team (HART), G.J. Motlhasedi (fieldworker), C. Lessing (research nurse), S. Péter and in-kind analyses of the national and international teams.
Conflict of interest: None declared.
References
- 1.Malan NT, Brits JS, Eloff FC, et al. The influence of acculturation on endocrine reactivity during acute stress in urban black males. Stress Med 1994;2:55–63. [Google Scholar]
- 2.Malan L, Schutte AE, Malan NT, et al. Specific coping strategies of Africans during urbanization: comparing cardiovascular responses and perception of health data. Biol Psych 2006;72:305–10. [DOI] [PubMed] [Google Scholar]
- 3.Malan L, Malan NT, Wissing MP, Seedat YK. Coping with urbanization: A cardiometabolic risk? Biol Psych 2008;79:323–28. [DOI] [PubMed] [Google Scholar]
- 4.Van Rooyen JM, Huisman HW, Eloff FC, et al. Cardiovascular reactivity in black South-African males of different age groups: The influence of urbanization. Ethn Dis 2002;12:69–75. [PubMed] [Google Scholar]
- 5.Schommer N, Helhammer DH, Kirschbaum C. Dissociation between reactivity of the hypothalamus-pituitary-adrenal axis and the sympathetic-adrenal-medullary system to repeated psychosocial stress. Psychosom Med 2003;65:450–60. [DOI] [PubMed] [Google Scholar]
- 6.Hamer M, Gibson EL, Vuononvirta R, Williams E, Steptoe A. Inflammatory and haemostatic responses to repeated mental stress: Individual stability and habituation over time. Brain Behav Immun 2006;20:456–59. [DOI] [PubMed] [Google Scholar]
- 7.von Känel R, Kudielka BM, Helfricht S, et al. The effects of aspirin and nonselective beta blockade on the acute prothrombotic response to psychosocial stress in apparently healthy subjects. J Cardiovasc Pharmacol 2008;51:231–38. [DOI] [PubMed] [Google Scholar]
- 8.Schlaich MP, Kaye DM, Lambert E, Sommervilee M, Socratous F, Esler MD. Relation between cardiac sympathetic activity and hypertensive left ventricular hypertrophy. Circulation 2003;108:560–65. [DOI] [PubMed] [Google Scholar]
- 9.Thayer JF, Lane RD. The role of vagal function in the risk for cardiovascular disease and mortality. Biol Psych 2007;74:224–42. [DOI] [PubMed] [Google Scholar]
- 10.Mc Ewen BS: Protection and damage from acute and chronic stress: allostasis and allostatic overload and relevance to the pathophysiology of psychiatric disorders. Ann N Y Acad Sci 2004;1032:1–7. [DOI] [PubMed] [Google Scholar]
- 11. TuTiempo. Climate. http://www.tutiempo.net/en/Climate/POTCHEFSTROOM/02-2008/683500.htm (17 July /2014, date last accessed).
- 12.Kohara K, Nishida W, Maguchi M, Hiwida K. Autonomic nervous function in non-dipper essential hypertensive participants: evaluation by power spectral analysis of heart rate variability. Hypertension 1995;26:808. [DOI] [PubMed] [Google Scholar]
- 13.Rogausch A, Kochen MM, Hennig J. Association between the Bcl glucocorticoid receptor polymorphism and smoking in a sample of patients with obstructive airway disease. Add Biol 2006;12:93–99. [DOI] [PubMed] [Google Scholar]
- 14.Sato N, Suzuki N, Sasaki N, et al. Corticotropin-releasing hormone receptor 1 gene variants in irritable bowel syndrome. PLoS One 2012;7:e42450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Obrist PA. Cardiovascular psychophysiology: A perspective. New York, NY: Plenum Press, 1981. [Google Scholar]
- 16.Kamarck TW, Lovallo WR. Cardiovascular reactivity to psychological challenge: conceptual and measurement considerations. Psychosom Med 2003;65:9–21. [DOI] [PubMed] [Google Scholar]
- 17.Liang YL, Teede H, Kotsoupoulos D, et al. Non-invasive measurements of arterial structure and function: repeatability, interrelationships and trial samples size. Clin Sci 1998;95:669–79. [DOI] [PubMed] [Google Scholar]
- 18.Hamer M, Malan L. Sympathetic nervous activity, depressive symptoms, and metabolic syndrome in black Africans: the Sympathetic Activity and Ambulatory Blood Pressure in Africans study. Stress 2012;15:562–68. [DOI] [PubMed] [Google Scholar]
- 19.Botha J, De Ridder H, Potgieter JC, Steyn HS, Malan L. Structural vascular disease in Africans: Performance of ethnic-specific waist circumference cut points using logistic regression and neural network analyses: the SABPA study. Exp Clin Endocrinol Diabetes 2013;121:441–47. [DOI] [PubMed] [Google Scholar]
- 20.Malan L, Hamer M, Schlaich MP, et al. Facilitated defensive coping, silent ischaemia and ECG left-ventricular hypertrophy: the SABPA study. J Hypertens 2012;30:543–50. [DOI] [PubMed] [Google Scholar]
- 21.Malan L, Hamer M, Schlaich MP, et al. Defensive active coping facilitates chronic hyperglycemia and endothelial dysfunction in African men: the SABPA study. Int J Cardiol 2013;168:999–1005. [DOI] [PubMed] [Google Scholar]
- 22.De Kock A, Malan L, Hamer M, Malan NT. Defensive coping and subclinical vascular disease risk – associations with autonomic exhaustion in Africans and Caucasians: the SABPA study. Atherosclerosis 2012;225:438–43. [DOI] [PubMed] [Google Scholar]
- 23.Hamer M, Malan L, Malan NT, et al. Objectively assessed health behaviors and sub-clinical atherosclerosis in black and white Africans: the SABPA study. Atherosclerosis 2011;215:237–42. [DOI] [PubMed] [Google Scholar]
- 24.Huisman HW, Schutte AE, Schutte R, et al. Exploring the link between cardiovascular reactivity and end-organ damage in African and Caucasian men: the SABPA study. Am J Hypertens 2013;26:68–75. [DOI] [PubMed] [Google Scholar]
- 25.Reimann M, Hamer M, Malan NT, et al. Autonomic responses to stress in Black versus Caucasian Africans: the SABPA study. Psychophysiology 2012;49:454–61. [DOI] [PubMed] [Google Scholar]
- 26.Uys AS, Malan L, Van Rooyen JM, Steyn HS, Reimann M, Ziemssen T. Attenuated NOx responses and myocardial ischemia, a possible risk for structural vascular disease in African men: the SABPA study. J Hum Hypertens 2014;28:438–43. [DOI] [PubMed] [Google Scholar]
- 27.Malan L, Hamer M, Schlaich MP, et al. Defensive coping facilitates higher blood pressure and early sub-clinical structural vascular disease via alterations in heart rate variability: the SABPA study. Atherosclerosis 2013, 227:391–97. [DOI] [PubMed] [Google Scholar]
- 28.Malan NT, von Känel R, Schutte AE, et al. Testosterone and acute stress are associated with fibrinogen and von Willebrand factor in African men: the SABPA study. Int J Cardiol 2013;168:4638–42. [DOI] [PubMed] [Google Scholar]
- 29.Von Känel R, Hamer M, Malan NT, Scheepers JD, Meiring M, Malan L. Procoagulant reactivity to laboratory acute mental stress in Africans and Caucasians, and its relation to depressive symptoms: the SABPA Study. Thromb Haem 2013;110:977–86. [DOI] [PubMed] [Google Scholar]
- 30.Botha J, Malan L, Potgieter JC, Steyn HS, De Ridder JH. Association of waist circumference with perception of own health in a group of urban African males and females: the Sympathetic Activity and Ambulatory Blood Pressure in Africans (SABPA) study. J Endocrinol Metab Diabetes SA 2012;17:106. [Google Scholar]
- 31.Hoebel S, Malan L, Botha J, Swanepoel M. Optimizing waist circumference cut-points for the metabolic syndrome and its components in a South African cohort at 3 year follow-up: the SABPA prospective cohort. Endocrine 2014. doi: 10.1007/s12020-014-0237-7. [DOI] [PubMed] [Google Scholar]
- 32.Amirkhan JH. A factor analytically derived measure of coping: The Coping Strategy Indicator. J Pers Soc Psychol 1990;59:1066–74. [Google Scholar]
- 33.Updegraff JA, Gable SL, Taylor SE. What makes experiences satisfying? The interaction of approach-avoidance motivations and emotions in well-being. J Pers Soc Psychol 2004;86:496–504. [DOI] [PubMed] [Google Scholar]
- 34.Alberti KGMM, Eckel RH, Grundy SM, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Arteriosclerosis Society; and International Association for the Study of Obesity. Circulation 2009;120:1640–45. [DOI] [PubMed] [Google Scholar]
- 35.Lichtman J, Froelicher ES, Blumenthal JA, Carney RM, Doering LV, Frasure-Smith N, et al. Depression as a risk factor for poor prognosis among patients with acute coronary syndrome: systematic review and recommendations. a scientific statement from the American Heart Association. Circulation 2014;129:1350–69. [DOI] [PubMed] [Google Scholar]