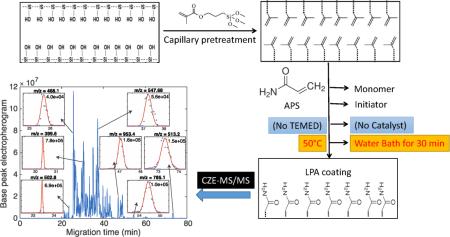
Abstract
Proteomic analysis using capillary zone electrophoresis (CZE) typically is performed with linear polyacrylamide (LPA) coated capillaries. These capillaries both minimize the adsorption of peptides and proteins to the inner wall of the capillary and decrease electroosmosis, which increases the separation capacity. LPA coating protocols were first reported by Hjerten in 1985. Conventional LPA production is based on the use of TEMED to catalyze the free-radical polymerization that couples acrylamide to a capillary wall that has been pretreated with γ-methacryloxypropyltrimethoxysilane. The treated capillary is filled with a mixture of monomer, TEMED, and ammonium persulfate; free radical polymerization forms the LPA coating. Over many years, we have observed significant variation in the electroosmotic properties of commercial LPA coated capillaries both along the capillary length and between lots. We believe this variation is due to differences in the time between initiation of the reaction and the filling of the capillary. Here, we report a simple method for the generation of very stable and reproducible coatings. In this protocol, the monomer mixture and an ammonium persulfate initiator are introduced into the capillary without TEMED initiator. The mixture is stable and does not begin polymerization at room temperature. The filled capillary is then heated in a water bath to initiate polymerization in a well-controlled manner. A mixture of four standard proteins was used to evaluate the coating performance. Compared with commercialized LPA capillaries, our LPA capillaries generate much better separation performance and superior protein peak shape in CZE analysis. We also analyzed an intact antibody (MW 150k) by CZE-MS with the new LPA capillary in triplicate runs. The intact antibody generated a Gaussian-shaped electrophoresis peak with 1.2% relative standard deviation in migration time and 8.5% in base peak intensity. An automated CZE-MS system was used to generate 97 successive separations of a BSA tryptic digest over a 145-hour period. Separation efficiency averaged over 100,000 theoretical plates across this period with no systematic variation. The LPA coating protocol had excellent batch-to-batch reproducibility with relative standard deviation in migration time < 7%, and in separation window < 1%.
Graphical Abstract
1. Introduction
Capillary zone electrophoresis (CZE) is attracting renewed attention as an alternative to reversed phase liquid chromatography (RPLC) for proteomics research.[1-7] Uncoated capillaries generate high electro-osmotic flow that leads to rapid separations, but with a short separation window that limits the number of peptide and protein identification in analysis of complex proteomes. Instead, deep proteomic analysis by CZE requires the use of capillaries that have had their interior coated with a hydrophilic polymer, which both suppresses EOF and minimizes peptide interaction with the wall.[8-10]
Linear polyacrylamide (LPA) coated capillaries are commonly used in CZE separations. [6, 7,11-15]. Typically the LPA coating is produced by covalently bonding acrylamide monomer to the inner wall of the fused silica capillary, as first described by Hjerten in 1985.[8] In this venerable protocol, the capillary is first treated with a vinylsilane to graft a double bond to the silica capillary wall. Next, a polymerization mixture is prepared by mixing the monomer (acrylamide), polymerization initiator (ammonium persulfate), and water in a tube. The solution is degased by N2 to remove oxygen, which can scavenge radicals. To initiate the reaction, tetramethylethylenediamine (TEMED) is added as a catalyst to the mixture; the polymerization reaction commences upon addition of TEMED. The polymerizing mixture must be introduced into the pretreated capillary rapidly after addition of TEMED. The time between TEMED addition and capillary filling critically affects the performance and reproducibility of the coating.[16,17] Usually, a special device is required to perform this step to generate good coating performance.[18]
In this manuscript, we report a novel preparation method for LPA coating that eliminates the use of TEMED or other catalyst. Our protocol eliminates the TEMED initiation. Instead, the degased polymerization solution containing acrylamide, ammonium persulfate, and deionized water is introduced into the capillary via vacuum. The capillary is then heated at 50 °C for 30 min to decompose the persulfate and generate radicals to initiate the reaction. Because the polymerization is initiated via heat after the mixture is introduced into the capillary, this polymerization process can be controlled precisely and is initiated simultaneously along the length of the capillary, leading to improved uniformity.
2. Materials and methods
2.1. Materials and chemicals
Acrylamide, ammonium persulfate, cytochrome c from bovine heart, myoglobin from equine skeletal muscle, beta-lactoglobulin from bovine milk, carbonic anhydrase from bovine erythrocytes, bovine serum albumin (BSA), bovine pancreas TPCK-treated trypsin, urea, ammonium bicarbonate (NH4HCO3), dithiothreitol (DTT), andiodoacetamide (IAA), and 3-(trimethoxysilyl)propyl methacrylate were purchased from Sigma-Aldrich (St. Louis, MO). Formic acid (FA) was purchased from Fisher Scientific (Pittsburgh, PA). Methanol and water were purchased from Honeywell Burdick & Jackson (Wicklow, Ireland). Uncoated (50 μm i.d./365 μm o.d.) and LPA coated capillaries (50 μm i.d./150 μm o.d.) were purchased from Polymicro Technologies (Phoenix, AZ ). Human/Mouse/Rat Activin A beta A subunit Antibody was purchased from R&D Systems(Minneapolis, MN)
2.2. Pretreatment of capillary
The capillary was flushed with 1 M hydrochloric acid for 30 min, water for 10 min, 1 M sodium hydroxide for 30 min, water for 10 min, and finally washed with methanol for 30 min by using a syringe pump at a flow rate of 2 μL/min. The capillary was then dried under a flow of nitrogen at room temperature for 4 hours. Next, 50 %( v/v) 3-(trimethoxysilyl) propyl methacrylate in methanol was flushed through the capillary for 10 min. Both ends of the capillary were sealed, and the filled capillary was incubated at room temperature for 24 h. Finally the capillary was rinsed by methanol for 20 min and dried under nitrogen. The pretreated capillary was stored at room temperature until use.
2.3. Preparation of Coating
40 mg of acrylamide was dissolved in 1 mL water. 2 μL of 5% (w/v) ammonium persulfate (APS) was added to the 500 μL of acrylamide solution. The mixture was vortexed for 30 s and degassed for 5 min using nitrogen. Then the mixture was introduced into 100 cm long pretreated capillary by vacuum. With both ends sealed, the capillary was incubated in a 50°C water bath for 30 min. The capillary was flushed with water to remove excess reagents, and was stored at room temperature before use. A ~1-mm length of the distal tip of the capillary was etched with HF for 90 min to make the outer diameter of the etched part ~70-μm; the detailed protocol for HF etching was described in Ref [21]. Because only outside of the capillary is in contact with the HF solution, the inner diameter of the capillary does not change. Caution – use appropriate safety protocols when handling HF.
2.4. Preparation of sample
A 0.5 mg/mL solution of BSA in 100 mM NH4HCO3 (pH 8.0) containing 8 M urea was denatured at 37 °C for 30 min, followed by standard reduction and alkylation with DTT and IAA [6,7]. Digestion was performed for 12 h at 37 °C with trypsin at a trypsin/protein ratio of 1/30 (w/w). The digests were desalted with a C18 SepPak column (Waters, Milford, MA), followed by lyophilization with a vacuum concentrator (Thermo Fisher Scientific, Marietta, OH). The dried samples were stored at −20 °C before use.
A mixture of standard proteins containing cytochrome c (0.05 mg/mL), myoglobin (0.15 mg/mL), beta-lactoglobulin (0.4 mg/mL) and carbonic anhydrase (0.15 mg/mL) dissolved in 10 mM NH4HCO3 (pH ~8.5) buffer was prepared for dynamic pH junction based CZE-MS/MS analysis.
Three 10 μg aliquots of intact antibody solution were desalted by using a C4 ZipTip (Millipore, Bedford, MA, USA). The sample was final eluted into a 20 μL of 35% acetic acid with 50% ACN solution for CZE-MS analysis.
2.5. CZE-ESI-MS
A PrinCE autosampler (Prince Technologies B.V., Netherlands) was used for automated sample injection and separation voltage control. A third-generation electrokinetically driven sheath–flow CE-MS nanospray interface was used to couple the separation with the mass spectrometer.[19-21] 5% acetic acid was used as the background electrolyte (BGE) and 0.5% formic acid with 10% methanol was used as the sheath buffer. The nanospray high voltage was supplied by a Spellman CZE 1000R power supply. The emitter was pulled in a P-1000 Sutter pipette puller to a 25 μm o.d. tip. The nanospray voltage was ~2 kV. The separation was performed at 25,000 V across 1 m of LPA-coated capillary (250 V cm−1). Sample injection was performed by pressure with 500 mbar for 0.2 min (injection volume 100 nL) for BSA digests and standard proteins and 500 mbar for 0.3 min for intact antibody injection. The LPA-coated separation capillary was coupled to a LTQ-XL (Thermo Fisher Scientific). Full MS scans were acquired over the 395−1900 m/z range for BSA digest analysis, 600-2000 for intact standard protein analysis and 600-4000 for intact antibody analysis.
3. Results and discussion
3.1. Preparation of LPA coating
Traditional LPA coating employs a mixture of acrylamide, TEMED and APS. [8, 16-18] Polymerization is initiated by addition of ammonium persulfate, and initiation and polymerization are catalyzed by TEMED. Typically, solutions of the three reagents are prepared individually. The solutions are degassed with either N2 or helium. APS is then mixed with the acrylamide solution. TEMED is then added to the mixture initiate the polymerization reaction; the solution is vortexed to mix the reagents, and the mixture is then introduced into the pretreated capillary. Using the traditional method polymerization is difficult to control because oxygen cannot be completely removed during TEMED addition step and the polymerization reaction is not reproducible.
APS alone as a thermal radical generator has been extensively used and reported in literature [22, 23]; however, to our knowledge, it has not been used in a LPA coating procedure. In our LPA coating preparation procedure, we eliminate the use of TEMED and instead use heat to trigger polymerization. The resulting capillaries have superior stability and reproducibility compared to commercial capillaries.
3.2. Stability
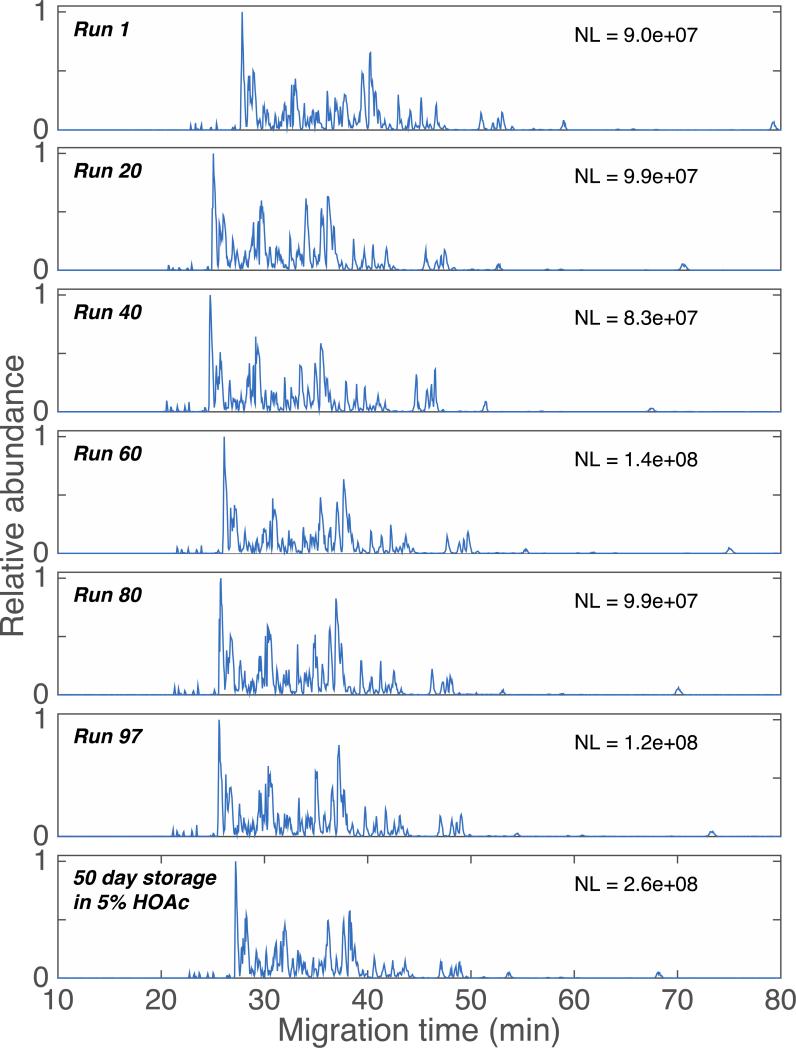
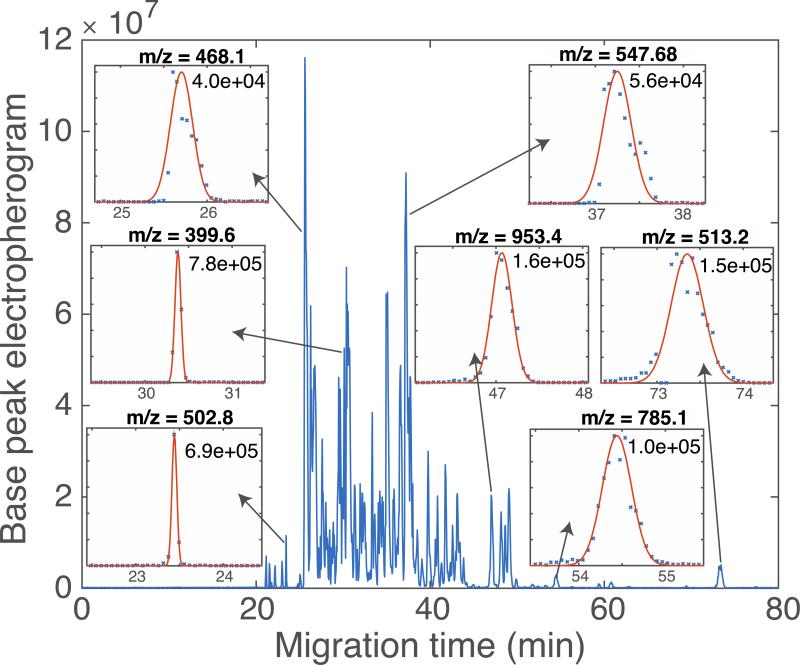
CZE-ESI-MS analysis of 1 mg/mL of the BSA digest and four standard proteins mixture were used to evaluate the LPA coated capillaries. The sample was dissolved in 10 mM NH4HCO3 to perform pH junction sample preconcentration.[24] An automated system was used to generate 97 consecutive separations of the BSA digest, Figure 1. In this analysis, the sample was injected every 90 minutes for 145 hours of continuous separation; no rinse or regeneration step was performed between injections. The separation profile and base peak intensity are reasonably reproducible. The variation in migration time averaged <4% (RSD) across the 145 hour run. The peak area varied by 35% (RSD), which is typical for pressure-driven CZE injection. A set of seven selected ion electropherograms of a BSA digest, generated after ~145 hours of continuous operation of a single coated capillary, is shown in Figure 2 as inserts. Plate counts range from 40,000 to 780,000, with a median plate count of 125,000, which is comparable to the best reports using commercial capillaries.[7,11] The normalization level and signal amplitude roughly doubled after storage, which presumably reflects differences in sample concentration. A set of six selected ion electropherograms (SIEs) generated a median of 110,000 theoretical plates, identical to that produced by the first 97 runs before storage. A t-test was used to test the hypothesis that the migration times observed in runs 1-97 had the same mean value as were generated using the stored capillary; we could not reject this hypothesis (p < 0.05) for four out of the six SIEs. The outstanding consistency of migration time and separation efficiency demonstrates the stability of this coating.
Figure 1.
Base peak electropherograms of BSA digests at selected runs and after storage for 50 days in 5% acetic acid. NL is the normalization level, the signal generated by the most intense peak in the base peak electropherogram.
Figure 2.
Base peak electropherogram of a BSA digest, generated after ~145 hours of continuous operation of a single coated capillary. Insets show selected ion electropherograms (SIEs) generated at the indicated m/z values. Data are shown as ‘x’. The smooth curves in the inserts are the result of an unsupervised least-squares fit of a Gaussian function to the SIEs. Theoretical plate counts are shown for each SIE, based on the regression analysis. Median number of theoretical plates = 125,000.
After the final injection, the capillary was stored for 50 days in 5% acetic acid, and another injection was performed. The bottom trace of Figure 1 presents the electropherogram generated after storage. The consistent separation window further demonstrates the stability of this coating.
3.3. Intact protein separation
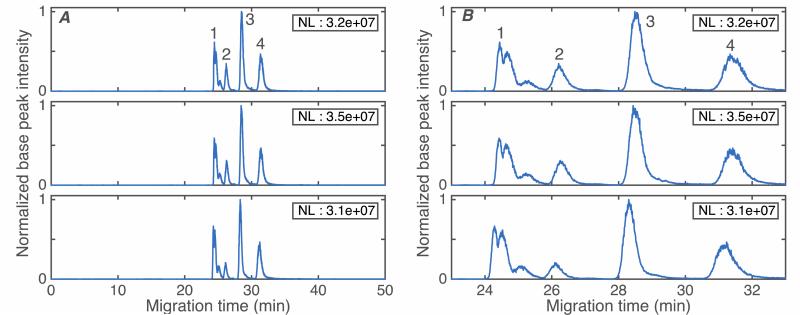
Figure 3 shows triplicate electropherograms of a mixture of four standard proteins. Beta-lactoglobulin and its natural variants are separated into three peaks (with molecular weight 18542, 18628, and 18952) using a capillary coated with the thermally-initiated polymerization procedure. We also performed a separation of the same sample using a 1-m long piece of commercially-coated LPA capillary, S-Fig1. The 1st injection generated a very small beta-lactoglobulin peak; instead most of proteins were adsorbed on the capillary wall. Subsequent injections generated increased peak intensities, but with poor separation performance and significant peak tailing. We assumed that active sites on the capillary wall bound to analyte, and that separation performance improved as those sites were covered by bound analyte. We observed that the separation performance improved after the commercial capillary was flushed with the protein mixture for 5 min, water for 10 min, and 5% acetic acid (BGE) for 10 min. As shown in bottom trace of S-Fig 1, the four proteins can be resolved after this procedure. Nevertheless, the separation performance of the LPA-coated capillary prepared with thermally-initiated polymerization is still much better than commercial LPA capillary. The resolution of cytochrome c and myoglobin is 1.2 for commercially coated capillary and 2.6 using a capillary coated using the thermally-initiated polymerization procedure (SFig 2).
Figure 3.
Base peak electropherograms of four standard proteins: 1 - beta-lactoglobulin, 2 - cytochrome c, 3 – myoglobin, and 4 - carbonic anhydrase in triplicate runs. Data are treated with a Lowess filter with Gaussian kernel and 10 point span. NL is the normalization level, which is the maximum value of the filtered electropherogram. A – full run. B – close-up of the standard proteins. Experimental conditions: BGE: 5% acetic acid, separation voltage: 250 v/cm, sample matrix: 10 mM NH4HCO3(pH 8), sample injection: 500 mbar for 0.2 min
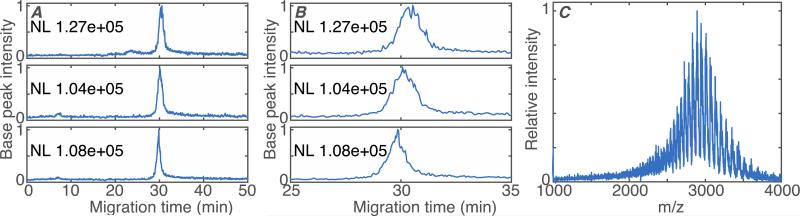
3.4. Analysis of intact antibody
A 1.4 mg/mL solution of intact antibody (MW 150 kDa) was analyzed by CZE-MS using a thermally-initiated LPA polymerization coated capillary. Figure 4A shows triplicate runs of intact antibody and Figure 4B shows the parent-ion spectrum. The electrophoretic peak is Gaussian, showed neither tailing nor fronting, and produced over 100,000 theoretical plates. The RSD of migration time and peak intensity are 1.2% and 8.5% respectively for the triplicate runs.
Figure 4.
Base peak electropherograms in triplicate runs (A – full run. B – closeup) and parent ion mass spectrum on intact antibody (C). For A and B, data are treated with a Lowess filter with Gaussian kernel and 10 point span. NL is the normalization level, which is the maximum value of the filtered electropherogram. For C, the parent ion mass spectra from 29.6 to 30.7 minutes were resampled at a common mass axis with 20,000 points between 1,000 and 4,000 Da. The spectra were then summed, the summed spectrum was treated with a Lowess filter with Gaussian kernel and 20-point span, and the averaged spectrum was normalized to unit height. Experimental conditions: BGE: 5% acetic acid, Separation voltage: 250 v/cm, sample matrix: 10 mM NH4HCO3(pH 8), sample injection: 500 mbar for 0.3 min
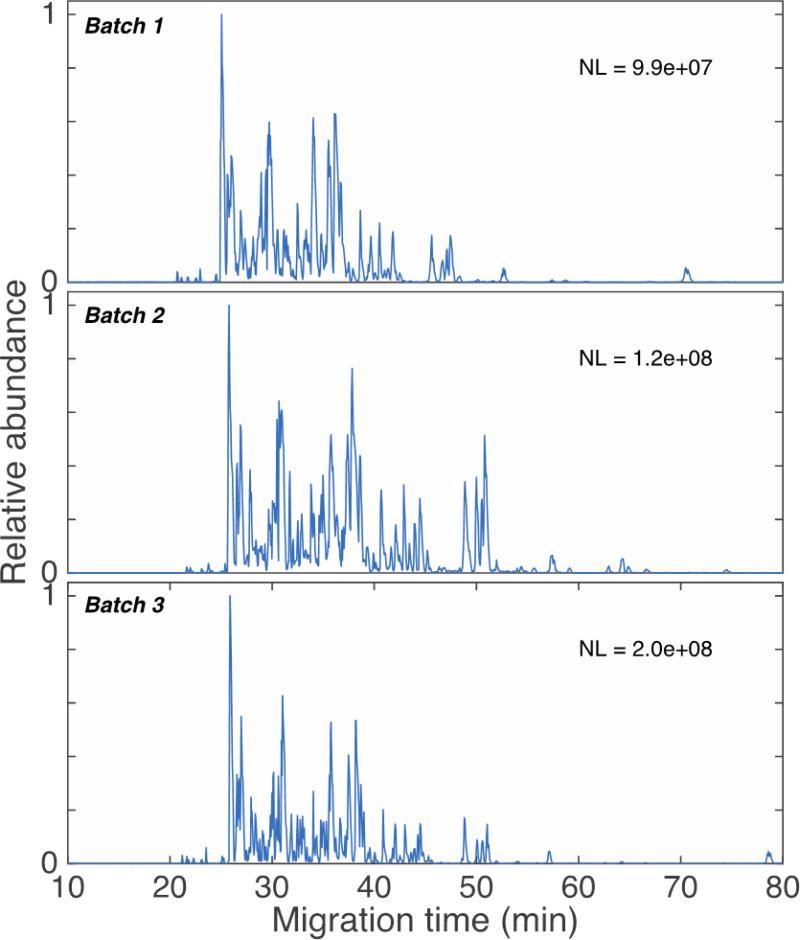
3.5. Reproducibility of the LPA coating
Finally, three batches of coating were prepared from two reels of uncoated capillaries, Figure 5. The top two traces show the BSA digests analysis on capillaries taken from the first two batches, which were made from the same capillary reel. The bottom trace shows the BSA digests analysis on a capillary taken from the 3rd batch, made from another reel. The separation profiles and separation windows are quite reproducible between batches. The relative standard deviation is 1% for the separation window, and <7% for migration time. Because the first two batches of LPA-coated capillaries were evaluated sequentially, they produced reproducible base peak intensity. The third LPA-coated capillary batch was evaluated 50 days after the first two, and difference in the base peak intensity is most likely due to changes in instrument conditions over this period.
Figure 5.
Base peak electropherograms of BSA digests generated with capillaries taken from three batches of LPA coated capillary. NL is the normalization level, the signal generated by the most intense peak in the base peak electropherogram.
Supplementary Material
Highlights.
Thermally-induced polymerization formed a linear polyacrylamide coating.
The coating was used for 145 hours with no performance degradation.
The coating provides outstanding resolution of peptides and proteins.
ACKNOWLEDGEMENTS
We thank Dr. William Boggess and Dr. Matthew Champion in the Notre Dame Mass Spectrometry and Proteomics Facility for their help with this project. This work was funded by the National Institutes of Health (Grant R01GM096767).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference
- 1.Jorgenson JW, Lukacs KD. Science. 1983;222:266–272. doi: 10.1126/science.6623076. [DOI] [PubMed] [Google Scholar]
- 2.Heemskerk AA, Deelder AM, Mayboroda OA. Mass Spectrom. Rev. 2014 May 23; doi: 10.1002/mas.21432. doi: 10.1002/mas.21432. [DOI] [PubMed] [Google Scholar]
- 3.Faserl K, Sarg B, Kremser L, Lindner H. Anal. Chem. 2011;83:7297–7305. doi: 10.1021/ac2010372. [DOI] [PubMed] [Google Scholar]
- 4.Wang Y, Fonslow BR, Wong CCL, Nakorchevsky A, Yates JR., 3rd Anal. Chem. 2012;84:8505–8513. doi: 10.1021/ac301091m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li Y, Champion MM, Sun L, Champion PAD, Wojcik R, Dovichi NJ. Anal. Chem. 2012;84:1617–1622. doi: 10.1021/ac202899p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhu G, Sun L, Yan X, Dovichi NJ. Anal. Chem. 2013;85:2569–2573. doi: 10.1021/ac303750g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sun L, Hebert AS, Yan X, Zhao Y, Westphall MS, Rush MJ, Zhu G, Champion MM, Coon JJ, Dovichi NJ. Angew. Chem. Int. Ed. 2014;53:13931–13933. doi: 10.1002/anie.201409075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hjertén SJ. Chromatogr. A. 1985;347:191–198. [Google Scholar]
- 9.Cifuentes A, de Frutos M, Santos JM, Diez-Masa JC, Chromatogr J. 1993;655:63–72. [Google Scholar]
- 10.Strege MA, Lagu AL. J. Chromatogr. 1993;630:337–344. [Google Scholar]
- 11.Busnel JM, Schoenmaker B, Ramautar R, Carrasco-Pancorbo A, Ratnayake C, Feitelson JS, Chapman JD, Deelder AM, Mayboroda OA. Anal. Chem. 2010;82:9476–9483. doi: 10.1021/ac102159d. [DOI] [PubMed] [Google Scholar]
- 12.Sun L, Knierman MD, Zhu G, Dovichi NJ. Anal. Chem. 2013;85:5989–5995. doi: 10.1021/ac4008122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yan X, Essaka DC, Sun L, Zhu G, Dovichi NJ. Proteomics. 2013;13:2546–2551. doi: 10.1002/pmic.201300062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao Y, Sun L, Champion MM, Knierman MD, Dovichi NJ. Anal. Chem. 2014;86:4873–4878. doi: 10.1021/ac500092q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haselberg R, de Jong GJ, Somsen GW. Anal. Chem. 2013;85:2289–2296. doi: 10.1021/ac303158f. [DOI] [PubMed] [Google Scholar]
- 16.Cifuentes A, Canalejas P, Diez-Masa JC. J. Chromatogr. A. 1999;830:426–438. doi: 10.1016/s0021-9673(98)00875-9. [DOI] [PubMed] [Google Scholar]
- 17.Huang X, Doneski LJ, Wirth MJ. Anal. Chem. 1998;70:4026–4029. doi: 10.1021/ac980231c. [DOI] [PubMed] [Google Scholar]
- 18.Gao I, Liu S. Anal. Chem. 2004;76:7179–7186. doi: 10.1021/ac049353x. [DOI] [PubMed] [Google Scholar]
- 19.Sun L, Zhu G, Zhang Z, Mou S, Dovichi NJ. J Proteome Res. 2015 Mar 27; doi: 10.1021/acs.jproteome.5b00100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wojcik R, Dada OO, Sadilek M, Dovichi NJ. Rapid Commun. Mass Spectrom. 2010;24:2554–2560. doi: 10.1002/rcm.4672. [DOI] [PubMed] [Google Scholar]
- 21.Sun L, Zhu G, Zhao Y, Yan X, Mou S, Dovichi NJ. Angew Chem Int. Ed. Engl. 2013;52:13661–13664. doi: 10.1002/anie.201308139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qin S, Qin D, Ford WT, Herrera JE, Resasco DE, Bachilo SM, Weisman RB. Macromolecules. 2004;37:3965–3967. [Google Scholar]
- 23.Thakur VK, Singha AS, Misra BN. J Appl. Polym. Sci. 2011;122:532–544. [Google Scholar]
- 24.Zhu G, Sun L, Yan X, Dovichi NJ. Anal. Chem. 2014;86:6331–6336. doi: 10.1021/ac5004486. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.