Figure 1.
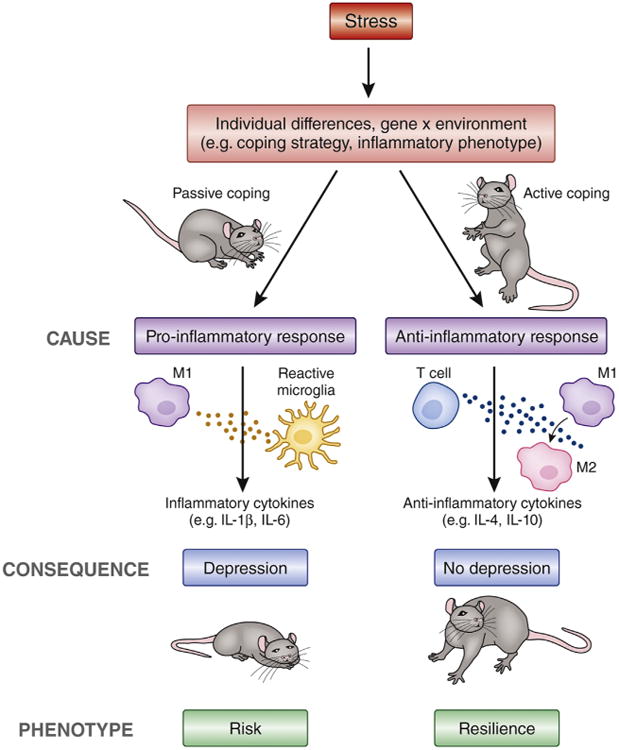
Inflammation mediates individual differences in risk and resilience to stress-induced depression. Individual differences in the response to stress (via gene × environment interactions) predict risk or resilience to the development of stress-induced behavioral changes. Inflammation has been shown in animal studies to be a pivotal causative factor of these individual differences. Animals who exhibit poor coping strategies exhibit exaggerated inflammatory responses (release of inflammatory cytokines, shift toward M1 macrophage phenotype, and activation of microglia) and are at risk for the development of depressive-like behavior. The causal role of inflammation in development of stress-induced depression was highlighted in the study by Wood et al. (8), which demonstrated that blocking interleukin-1β before stress had no effect on individual differences in coping strategy but prevented individual differences in the induction of depressive-like behavior. Furthermore, resilient animals displayed anti-inflammatory responses to stress, consistent with a previously established protective role of the acquired immune system (T cells) and anti-inflammatory cytokines in promoting a shift toward an M2-like macrophage phenotype, reduced inflammation in the brain, and prevention of stress-induced depression. IL, interleukin.
