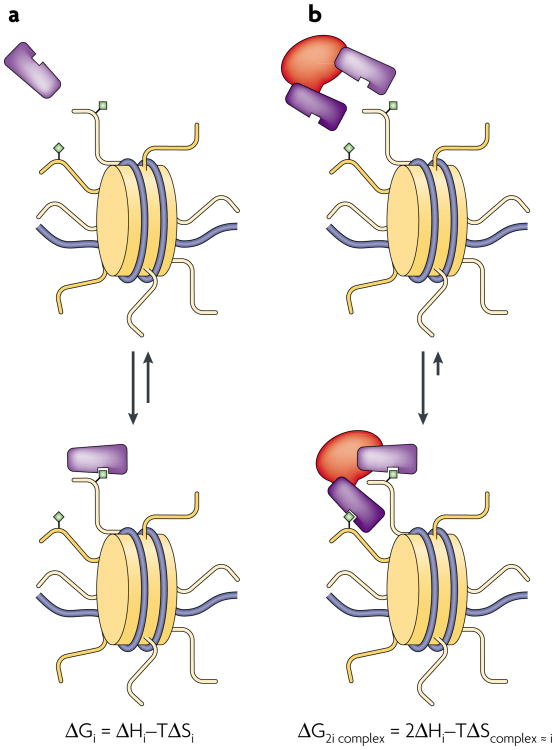
Figure 1. Thermodynamics of multivalent binding.
a | Monovalent association of a hypothetical effector module (purple) to a chromatin substrate (yellow tail with green diamond) is simplistically compared to a bivalent association of the same effector in a complex, representing the lowest order of multivalent interactions. The change in free energy ΔGi for the monovalent system undergoing binding is indicated by the change in enthalpy ΔHi minus the change in entropy ΔSi, scaled by the temperature (T). b | By tethering the two effector modules, the entropy term may be, to a first approximation, similar for each of the binding equilibria in panels a and b (TΔScomplex ≈ TΔSi). For our purposes, this example assumes that hydrogen-bonding electrostatic interactions dominate and desolvation is negligible, so that ΔS for the system will be negative. Thus, the entropic penalty to binding is lessened approximately twofold by pre-organizing the effector domains in a complex (TΔScomplex ≈ TΔSi), while the enthalpy of the bivalent domain interaction is effectively double that of the monovalent case (∼2ΔHi, if enthalpic penalties due to the strain induced by bivalent binding are negligible). In this manner, the reduced net entropy loss for the binding process can be a significant determinant of free energy, especially in low-binding enthalpy regimes. Losses of entropy on the substrate side would be expected to be minimal due to the low intrinsic rotational and translational freedom of chromatin; however, conformational entropy losses here are assumed to be negligible for simplicity.