Table 1.
Reaction condition optimization.a
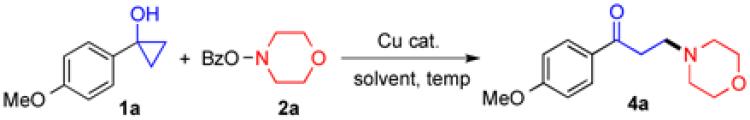
| ||||
|---|---|---|---|---|
|
| ||||
| entry | Cu cat. | solvent | temp (°C) | yield (%)a |
|
| ||||
| 1 | CuI | THF | 80 | 85b |
| 2 | CuI | THF | 80 | 88 |
| 3 | CuI | toluene | 80 | 75 |
| 4 | CuI | DCE | 80 | 65 |
| 5 | CuI | 1,4-dioxane | 80 | 82 |
| 6 | CuI | MeCN | 80 | 90 |
| 7 | - | MeCN | 80 | 0 |
| 8 | CuI | MeCN | rt | 39 |
| 9 | CuI | MeCN | 50 | 93 |
| 10 | CuCl | MeCN | 50 | 88 |
| 11 | CuBr | MeCN | 50 | 98 |
| 12 | CuBr | MeCN | 50 | 93b |
| 13 | CuBr | MeCN | 50 | 80c |
| 14 | CuBr | MeCN | 50 | traced |
General reaction conditions: A solution of 1a (0.15 mmol), 2a (0.1 mmol), and Cu(I) catalyst (0.1 equiv) were stirred until no more starting material left. The reaction process was monitored by thin-layer chromatography. Isolated yield from flash chromatography was given;
1/1 of 1a/2a (0.1 mmol);
1a (0.1 mmol) and 2a (0.15 mmol);
Phenanthroline (0.1 equiv).
