Abstract
Introduction
The pathogenesis of periapical lesions is determined by the balance between host pro-inflammatory immune response and counteracting anti-inflammatory and reparative responses, which include regulatory T cells (Tregs) as potential immunoregulatory agents. In this study, we investigated (in a cause-and-effect manner) the involvement of CCL22-CCR4 axis in Tregs migration to the periapical area and the role of Tregs in the determination of outcomes in periapical lesions.
Methods
Periapical lesions were induced in C57Bl/6 (WT) and CCR4KO mice (pulp exposure and bacterial inoculation), and treated with anti-GITR to inhibit Tregs function or alternatively with CCL22-releasing, PLGA particles to induce site-specific migration of Tregs. Post treatment, lesions were analyzed for Tregs influx and phenotype, overall periapical bone loss and inflammatory/immunological and wound healing markers expression (analyzed by RealTimePCRarray).
Results
Tregs inhibition by anti-GITR or CCR4 depletion results in a significant increase in periapical lesions severity, associated with upregulation of proinflammatory, Th1, Th17 and tissue destruction markers in parallel with decreased Tregs and healing markers expression. The local release of CCL22 in the root canal system resulted in the promotion of Tregs migration in a CCR4-dependent manner, leading to the arrest of periapical lesions progression, associated with downregulation of pro-inflammatory, Th1, Th17 and tissue destruction markers in parallel with increased Tregs and healing markers expression.
Conclusions
Since the natural and CCL22 induced Tregs migration switch active lesion into inactivity phenotype, Tregs chemoattractant may be a promisor strategy for the clinical management of periapical lesions.
Keywords: Apical lesions, cytokines, regulatory T cells, T helper, wound healing
INTRODUCTION
Pathogenesis of periapical lesions involves a complex host inflammatory immune response to the bacterial infection of the root canal system, which ultimately drives the destruction of periapical tissue (1). The breakdown of soft and mineralized tissues surrounding the root apex is triggered by a series of host mediators, which independently or cooperatively mediate an increased proteolytic activity and the activation of bone resorption mechanisms (1–5).
However, host response regulatory mechanisms activated along lesion development can convert active lesion into an inactive phenotype and consequently arrest or limit progression of tissue destruction (6). Protective mechanisms involve certain T helper (Th) subsets, mesenchymal stem cells and suppressors of cytokine signaling, which collectively are thought to dampen the tissue destructive pathways while boosting healing mechanisms (6–10). Within the potentially protective Th subsets, accumulating evidence points to the involvement of regulatory T cells (Tregs), as potential determinants of lesions outcome (6, 11–13).
Tregs comprise a CD4+CD25+ T cell subpopulation that specifically suppresses the activation, proliferation and pro-inflammatory effector function of activated conventional T cells (14, 15),(6, 11–13). Tregs were identified in human and experimental periapical lesions by the expression of phenotypic markers FOXp3, GITR, CD103 and CD45RO, as well by the functional markers CTLA-4, IL-10, TGF-β, which are associated with Tregs suppressive function (6, 11, 12, 16). Indeed, the presence of Tregs in periapical lesions accounts for the attenuation of local host inflammatory immune responses (8, 17–19). Accordingly, decreased expression of Treg phenotypic and functional markers, as well its impaired function due increased FOXp3 methylation, are characteristic features of progressive human periapical lesions (20). Migration of Tregs has been associated with the chemotactic cytokine CCL22 as well as decreased severity of experimental periapical lesion in rats (21). Accordingly, CCL22 interaction with the receptor CCR4 appears to control Treg migration into mice periodontal tissues and consecutively suppresses local inflammatory bone loss (22).
Regardless, studies to date only support a theoretical protective role exerted by Tregs in the control of periapical lesions severity, which remains to be definitely confirmed in a cause-and-effect manner. For this reason, we investigated the phenotypic features and kinetics of Tregs migration along experimental periapical lesions development in mice. In addition, the mechanisms underlying Tregs migration and function in periapical environment were investigated by its inhibition (with anti-GITR treatment) or chemoattraction (via CCL22/CCR4 axis).
MATERIAL AND METHODS
Experimental groups
Experimental groups comprised 8-week-old male C57BL/6 wild-type (WT) and CCR4 (CCR4KO) mice, treated with anti-GITR or with CCL22 releasing particles (CCL22p) (22, 23). Anti-GITR antibodies were prepared from hybridomas grown in nude mice, as previously described (26). CCL22 releasing particles were prepared by mixing an aqueous solution containing CCL22 and bovine serum albumin (BSA) with PLGA; followed by sonication, homogenization, evaporation and lyophilization, as previously described (23). Over the course of the study, the mice were maintained in the animal facilities of USP and fed with standard solid mice chow (Nuvital, Curitiba, PR, Brazil) and sterile water. The experimental protocol was approved by the local Institutional Committee for Animal Care and Use follows the principles of the Guide for the Care and Use of Laboratory Animals and EU Directive 2010/63/EU for animal experiments.
Experimental periapical lesions & treatments
Periapical lesion induction and quantification was performed as previously described (3, 7). Mice (N=5/time/group) were anesthetized, mandibular first molars dental pulp was exposed with a carbide bur in a slow-speed handpiece followed by with endodontic pathogenic bacterial strains (Porphyromonas gingivalis ATCC33277, Prevotella nigrescens ATCC33563, Actinomyces viscosus ATCC91014, and Fusobacterium nucleatum ATCC10953) inoculation (3, 7). Tregs function was inhibited by treatment with purified mAb anti-GITR (or control rat IgG) 500µg/mice i.p. injection, as previously described (24). Tregs migration was induced by the CCL22-releasing PLGA microparticles (or control blank particles) (22, 23), injected (5µL of PBS/CMC solution containing 25mg/mL particles) in the root canal system at day 3 after bacterial inoculation. Animals were killed by cervical displacement after 0, 3, 7, 14 and 21 days of infection, the jaws were dissected, and independent samples were prepared for histomorphometric (right molars) or molecular (left molars) analysis. Results are depicted as the area of periapical space (9), measured in HE stained longitudinal 5-mm-thick sections by using ImageJ (NIH, Bethesda, MD), being the area increase over time representative of lesion development.
Gene Expression and ELISA
RealTimePCR array reactions were performed as previously described (7). The extraction of total RNA from periapical tissues was performed with the RNeasyFFPE kit (Qiagen Inc, Valencia, CA), followed by integrity analysis with 2100Bioanalyzer (Agilent Technologies, Santa Clara, CA) and complementary DNA synthesis (Superscript III, Invitrogen Corporation, Carlsbad, CA, USA); all performed according to the manufacturers’ instructions. RealTimePCR array was performed in a Viia7 instrument (LifeTechnologies, Carlsbad, CA) using a custom panel for gene expression profiling (SABiosciences, Frederick, MD), analyzed by the RT2profiler software (SABiosciences) for normalizing target genes expression levels by constitutive genes (GAPDH, ACTB, Hprt1) and the control group, Gene expression levels are expressed as fold change relative to the control group; as previously described (9). Measurements of cytokines IL-10, TGF-b, TNF and RANKL in periapical lesions was performed by ELISA; as previously described (22), by commercially available kits (R&D Systems, Minneapolis, USA) according to the manufacturer’s instructions. The results are expressed as picograms of cytokine (±SD) per milligram of periapical tissue.
Isolation and analysis of leukocytes from periapical tissues
Isolation and characterization of lesions’ Tregs were performed as described previously (22). Whole periapical tissues of molars apex were initially incubated in RPMI-1640 with 0·28 Wunsch units/ml of liberase blendzyme CI (Roche, Basel, Switzerland), and then processed with 0·05% DNase (Sigma-Aldrich, Steinhein, Germany) using Medimachine (BDbiosciences, San Diego, CA, USA); followed by cell viability analysis (Trypan blue) and cell count (Neubauer chamber). Cells were incubated with optimal dilution fluorochrome-conjugated antibodies against CD4 (FITC, clone GK1.5, dilution 1:200), FOXp3 (Alexa488, R16-715, 1:100), CCR4 (PE, 1G1, 1:100), CCR5, (PE, C34-3448, 1:100), CCR7, (PE, 4B12, 1:50), CCR8, (PE, 1055c, 1:50), CXCR3, (PE, 1C6/CXCR3, 1:100), RANKL, (PE, IK22-5, 1:100), IL-10, (PE, JES5-16E3, 1:100), TGF-b and (PE, TW7-16B4, :200), CTLA-4 (PE, UC10-4F10-11, 1:200) (BD Biosciences) and then analyzed by flow cytometry (FACScan and CellQuest; BD Biosciences). Results represent the number of cells ± SD in the periapical tissues of each mouse, normalized by the tissue weight, or the number of positive cells for each marker in CD4+FOXp3+ subpopulation.
Statistical analysis
Data are presented as means ± SD, and the statistical significance between the groups was analyzed by Kruskal-Wallis followed by Dunns post test, or by and Mann-Whitney test, both performed with GraphPad Prism 5.0 software (GraphPad Software Inc., San Diego, CA). PCRarray data was analyzed by the Mann-Whitney test followed by Benjamini-Hochberg test. Values of P<0.05 were considered statistically significant.
RESULTS
Tregs influence experimental periapical lesions outcome
The induction of experimental periapical lesions generated increasing Treg (CD4+FOXP3+) migration over time, while conventional T cells (CD4+FOXP3−) counts remained in stable counts after 7d time point (Fig.1A). The development of experimental lesions in WT mice was similar to what was observed in previous reports (7), and inhibition of Tregs with anti-GITR resulted in a significant increase in the lesions at 14d and 21d time points (Fig.1B), as well increased lesion evolution rate (Fig.1C). Additionally, Tregs counts negatively correlates with lesion evolution patterns (Fig.1D). Treatment with anti-GITR was associated with increased expression of pro-inflammatory cytokines and tissue destructive mediators (IL1B, IL17, TNF-a, IFN-g, MMP2, RANKL) as well as decreased expression of Tregs markers (IL-10, TGF-b, CTLA-4) and wound healing/inactive lesions markers (CTGF, FGF7, ITGA4, SERP1, VTN) (Fig.1E). ELISA analysis confirmed the molecular analysis, showing the upregulation of TNF and RANKL in parallel with the downregulation of IL-10 and TFG-b by anti-GITR treatment (Fig.1F).
Figure 1. Tregs migration kinetics and its impact on the periapical bone loss and in the expression of inflammatory/immunological and healing markers in experimental periapical lesions in mice.
C57Bl/6 (WT) mice were submitted to an experimental periapical lesion inducing protocol (pulp exposure and bacterial inoculation) and treated (or not) with anti-GITR to inhibit Tregs function. Samples from experimental and control groups were collected for histomorphometric and molecular analysis, and evaluated for: (A) Tregs (CD4+FOXp3+) cells count in periapical tissues analyzed by flow cytometry, at 0, 3, 7, 14 and 21 days post infection; depicted as the cell number ×104; (B) periapical lesion development, presented as periapical space area (mm2) increase after lesions induction, measured with ImageJ software in HE stained histological sections, or presented as (C) the lesion evolution index (fold increase in specific time intervals); the (D) correlation between the Tregs (CD4+FOXp3+) cells count and the lesion evolution index, performed with the data from WT group; (E) the expression of inflammatory/immunological and wound healing markers at 21d time point, measured quantitatively by RealTimePCRarray, presented as fold change relative to the control group after normalization by constitutive genes (GAPDH, ACTB, Hprt1) expression levels; and (F) cytokine levels in periapical lesions, measured by ELISA, presented as cytokine pg/mg of periapical tissue. In A panel, different letters represent statistically significant differences among the different time points within its respective groups (P<0.05; One-way ANOVA, Bonferroni post-test). In A, B, C, E and F panels, asterisks (*) represent statistically significant differences (P<0.05; One-way ANOVA, Bonferroni post-test) between the indicated group/time point vs control group, and the hashtag (#) represent statistically significant differences (P<0.05; One-way ANOVA, Bonferroni post-test) between the indicated group/time point vs WT group.
Involvement of CCR4 and CCL22 in Tregs migration and lesions outcome
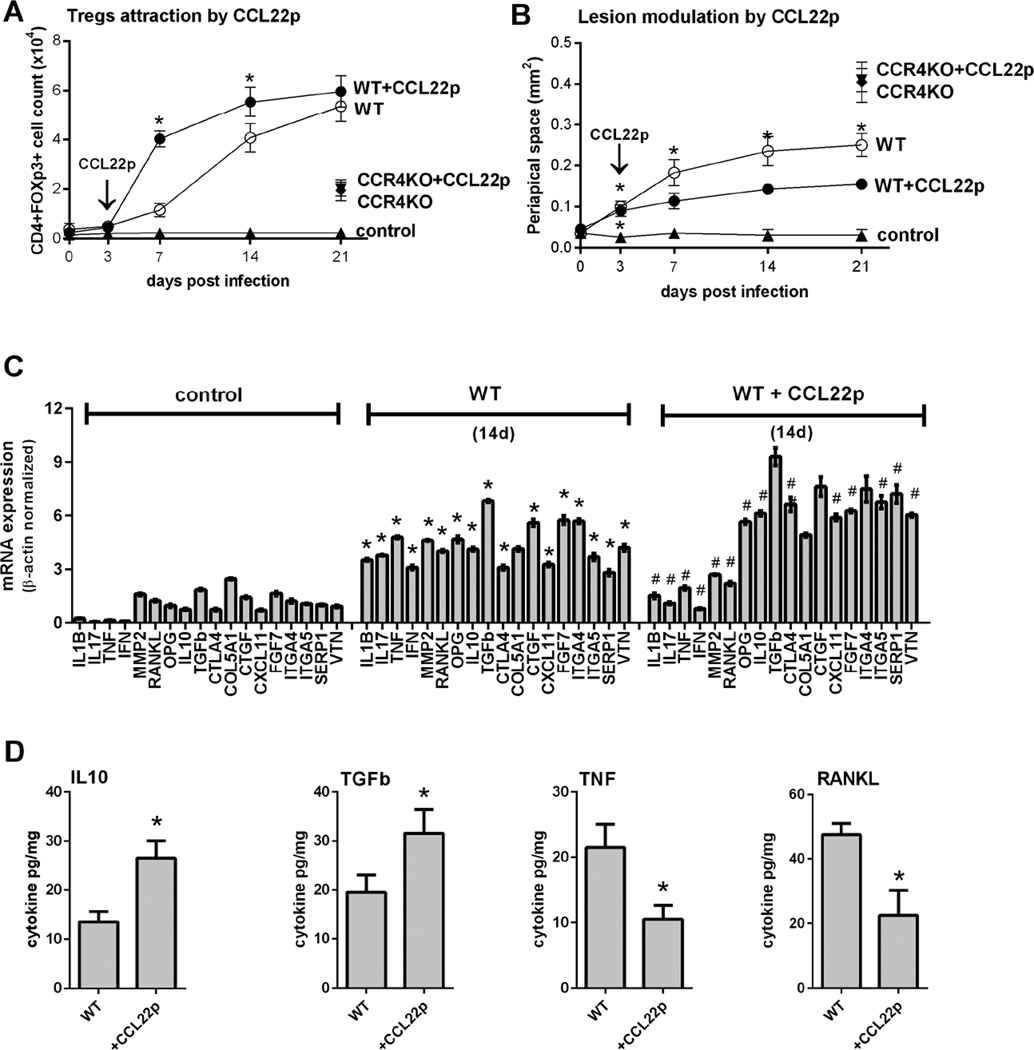
Phenotypic analysis demonstrates that periapical lesions’ Tregs (CD4+FOXp3+cells) express high levels of the chemokine receptor CCR4 and its classic markers IL-10, TGF-b, CTLA-4, and low but significant levels of CCR5 and CCR8 (Fig.2A). In the absence of CCR4 (CCR4KO strain), the migration of Tregs was significantly impaired (Fig.2B) and the severity of lesions increased (Fig.2C). Molecular analysis of CCR4KO mice lesions demonstrated an increased expression of pro-inflammatory cytokines and tissue destructive mediators (IL1B, IL17, TNF-a, IFN-g, MMP2, RANKL) and decreased the expression of Treg-associated markers (IL-10, TGF-b, CTLA-4) as well as markers of wound healing/inactive lesions (OPG, CTGF, CXCL11, FGF7, ITGA4, SERP1) (Fig.2D). ELISA analysis confirmed data obtained via RealTimePCR, suggesting that TNF and RANKL were upregulated in CCR4KO mice in parallel with the downregulation of IL-10 and TFG-b (Fig. 2E). The injection of CCL22-releasing particles in the root canal system appears to be effective in promoting Tregs migration (Fig.3A) and reducing lesion progression (Fig.3B) in a CCR4-dependent manner. From the molecular viewpoint, CCL22 treatment resulted in a decreased expression of pro-inflammatory cytokines and tissue destructive mediators (IL1B, IL17, IFN-g, TNF-a, MMP2, RANKL) and increased the expression of Tregs markers (IL-10, TGF-b, CTLA-4) and wound healing/inactive lesions markers (OPG, CXCL11, FGF7, ITGA5, SERP1, VTN) (Fig.3C). Similarly, ELISA analysis confirmed that CCL22-releasing particles mediate the downregulation of TNF and RANKL levels in parallel with the upregulation of IL-10 and TFG-b in the lesions (Fig.3D).
Figure 2. The role of CCR4 in Tregs migration kinetics and its impact on the periapical bone loss and in the expression of inflammatory/immunological and healing markers in experimental periapical lesions in mice.
C57Bl/6 (WT) and CCR4KO mice were submitted to an experimental periapical lesion inducing protocol (pulp exposure and bacterial inoculation). Samples from WT and CCR4KO groups were collected for histomorphometric and molecular analysis, and evaluated for:(A) the phenotype of and Tregs (CD4+FOXp3+) from periapical lesions, evaluated by flow cytometry and depicted as the number of positive cells for each marker; (B) Tregs (CD4+FOXp3+) cells count in periapical tissues analyzed by flow cytometry, at 21 days post infection, depicted as the cell number ×104; (C) periapical lesion development, presented as periapical space area (mm2) increase after lesions induction, measured with ImageJ software in HE stained histological sections; (D) the expression of inflammatory/immunological and wound healing markers at 21d time point, measured quantitatively by RealTimePCRarray, presented as fold change relative to the control group after normalization by constitutive genes (GAPDH, ACTB, Hprt1) expression levels, and (E) cytokine levels in periapical lesions, measured by ELISA, presented as cytokine pg/mg of periapical tissue. Asterisks (*) represent statistically significant differences (P<0.05; One-way ANOVA, Bonferroni post-test) between the indicated group/time point vs control group, and the hashtag (#) represent statistically significant differences (P<0.05; One-way ANOVA, Bonferroni post-test) between the indicated group/time point vs WT group.
Figure 3. Involvement of CCL22 in Tregs migration kinetics and its impact on the periapical bone loss and in the expression of inflammatory/immunological and healing markers in experimental periapical lesions in mice.
C57Bl/6 (WT) and CCR4KO mice were submitted to an experimental periapical lesion inducing protocol (pulp exposure and bacterial inoculation) and treated (or not) with CCL22 releasing PLGA particles to induce Tregs migration, by the direct injection of the particles at root canal system at 3d time point. Samples from WT and CCR4KO strains from control and experimental groups were collected for histomorphometric and molecular analysis, and evaluated for: (A) Tregs (CD4+FOXp3+) cells count in periapical tissues analyzed by flow cytometry at 0, 3, 7, 14 and 21 days post infection; depicted as the cell number ×104; (B) periapical lesion development, presented as periapical space area (mm2) increase after lesions induction, measured with ImageJ software in HE stained histological sections; (C) the expression of inflammatory/immunological and wound healing markers at 21d time point, measured quantitatively by RealTimePCRarray, presented as fold change relative to the control group after normalization by constitutive genes (GAPDH, ACTB, Hprt1) expression levels, and (D) cytokine levels in periapical lesions, measured by ELISA, presented as cytokine pg/mg of periapical tissue. Asterisks (*) represent statistically significant differences (P<0.05; One-way ANOVA, Bonferroni post-test) between the indicated group/time point vs control group, and the hashtag (#) represent statistically significant differences (P<0.05; One-way ANOVA, Bonferroni post-test) between the indicated group/time point vs WT group.
DISCUSSION
Pathogenesis of periapical lesions is determined by the balance between the host pro-inflammatory immune response and the counteracting anti-inflammatory and reparative responses (1, 5, 7, 8), which include Tregs as potential immunoregulatory agents. In this study, we investigated (in a cause-and-effect manner) the involvement of CCL22-CCR4 axis in migration of endogenous Tregs to periapical area and their subsequent role in the control of outcomes in periapical lesions.
Our results demonstrate that migration of Tregs (CD4+FOXP3+ cells) into periapical lesions is temporally and functionally associated with the attenuation of the progression of these lesions. In WT mice, the increasing number of Tregs in periapical lesions is temporally associated with the conversion of the lesion phenotype from active into inactive, a previously described phenomenon (9). Additionally, the inhibition of Treg function (with anti-GITR) prevents the establishment of the inactive/stable status resulting in the exacerbation of lesions severity. Accordingly, Tregs were previously suggested to attenuate inflammatory osteolysis in arthritis and periodontitis models (25, 26). Regarding the mechanisms underlying Tregs protective function in periapical milieu, our data suggests that Treg inhibition results in a decreased expression of IL-10, TGF-b, CTLA-4, typically associated with Treg-mediated immunosuppressive properties (22, 23). Indeed, Treg inhibition appears to imbalance host response, as demonstrated by the increased the expression of pro-inflammatory and Th1 and Th17 cytokines, previously associated with periapical lesions progression (1). Lesions exacerbation in the absence of functional Tregs is supposed to be due the upregulation of catabolic factors such as MMP2 and the osteoclastogenic factor RANKL, in parallel with decreased expression of OPG and wound healing related factors (COL5A1, CTGF, CXCL11, FGF7, ITGA4, ITGA5, SERP1, VTN). Accordingly, in the absence of Tregs the overall molecular profile of periapical lesions match with the profile previously described to active lesions, while the gene expression profile of lesions populated by active Tregs resemble the profile of inactive/stable lesions (7, 10).
Considering that Tregs migration into the periapex arrests lesions progression, we evaluated the mechanism of chemoattraction of Tregs into the lesions. Our data demonstrate that Tregs extracted from the lesions express the chemokine receptor CCR4, but not CCR5, CCR7, CCR8 and CXCR3 (Fig. 2A). Interestingly, while Tregs can express different chemokine receptors varying according to the developmental stage and the environment where they undergo activation, CCR4 seems to be essential to its migration to mucosal and periodontal tissues (22, 27, 28). Indeed, our data demonstrate the essential role of CCR4 in Tregs migration since CCR4KO mice presents a significant decrease in CD4+FOXP3+ cells count in the aggravated periapical lesions observed in this strain. Similarly, CCR4KO mice develops an increased periodontitis severity in response to experimental infection due impaired Treg migration (22). Importantly, the gene expression profile in CCR4KO-derived periapical lesions is very similar to that observed in the lesions from WT mice treated with anti-GITR (i.e. increased expression of pro-inflammatory cytokines and tissue destructive mediators and decreased the expression of Tregs markers and wound healing markers), reinforcing that the compromised Treg migration accounts for the aggravated lesions of CCR4KO mice. Indeed, adoptive transfer of CCR4+ Tregs restore original host response phenotype of CCR4KO mice in experimental periodontitis (22). Similarly, in rats’ experimental periapical lesions, the chemokine CCL22, a CCR4 ligand, was chronologically alleged to mediate Treg appearance and regulatory activity (21). Our results confirm this hypothesis, since CCL22 release in the root canal system (by means of a previously developed degradable PLGA microparticles containing recombinant CCL22 (29)) effectively promotes Treg migration and arrests progression of the lesions. Importantly, the protective effect of CCL22-releasing particles was proven CCR4-dependent, since such treatment was ineffective in CCR4KO strain.
Interestingly, while the CCL22/CCR4/Tregs axis compromises anti-tumoral immunity, and its inhibition with anti-CCR4 antibody (Mogamulizumab) can improve therapeutic protocols (30, 31), chemoattraction of Tregs via CCL22/CCR4 was demonstrated to be a promising therapeutic strategy to different chronic inflammatory conditions (23, 32, 33). Indeed, it is important to consider that the approval for polymeric controlled release systems for clinical use in humans, along the biological effectiveness of extremely miniscule amounts of chemokine (ng/kg total body weight), reinforces the potential safety and affordability of the proposed strategy and may enable the translation to clinical reality (34, 35).
Therefore, considering that the CCL22-mediated Tregs migration switches active periapical lesions into an inactivity phenotype, Tregs chemoattractant may be a promising strategy for the clinical management of periapical lesions.
Conclusion
Our results demonstrate that Tregs are active immunosuppressive (and even pro-healing) agents in periapical granuloma pathogenesis. Treg chemoattraction to the periapical area is mediated by the CCL22/CCR4 axis. Treatments oriented on recruitment of endogenous Tregs can be potentially explored as a clinical strategy for management of periapical lesions.
Highlights.
Regulatory T cells (Tregs) migration into periapex is temporally associated with the arrest of lesions progression
Tregs inhibition (by anti-GITR) results in the exacerbation of host response and increased periapical lesions severity
Tregs migration into periapex is mediated by the chemokine receptor CCR4
Tregs migration into periapex is mediated by the chemokine CCL22, a CCR4 ligand
The chemoattraction of Tregs by CCL22-releasing PLGA particles arrest of periapical lesions progression, downregulation of host response and increased healing/regeneration markers expression
ACKNOWLEDGMENTS
The authors would like to thank Daniele Ceolin, Patricia Germino and Tania Cestari for their excellent technical assistance. This study was supported by grants and scholarships form FAPESP (2012/15133-3 and 2013/05994-4), NIH-NIDCR (1R01DE021058-01 A1 and 1R56DE021058-01) and Wallace H. Coulter Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors deny any conflicts of interest related to this study.
References
- 1.Graves DT, Oates T, Garlet GP. Review of osteoimmunology and the host response in endodontic and periodontal lesions. J Oral Microbiol. 2011;3 doi: 10.3402/jom.v3i0.5304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Letra A, Ghaneh G, Zhao M, Ray H, Jr, Francisconi CF, Garlet GP, et al. MMP-7 and TIMP-1, new targets in predicting poor wound healing in apical periodontitis. J Endod. 2013;39(9):1141–1146. doi: 10.1016/j.joen.2013.06.015. [DOI] [PubMed] [Google Scholar]
- 3.Fukada SY, Silva TA, Saconato IF, Garlet GP, Avila-Campos MJ, Silva JS, et al. iNOS-derived nitric oxide modulates infection-stimulated bone loss. J Dent Res. 2008;87(12):1155–1159. doi: 10.1177/154405910808701207. [DOI] [PubMed] [Google Scholar]
- 4.Menezes R, Garlet TP, Letra A, Bramante CM, Campanelli AP, Figueira Rde C, et al. Differential patterns of receptor activator of nuclear factor kappa B ligand/osteoprotegerin expression in human periapical granulomas: possible association with progressive or stable nature of the lesions. J Endod. 2008;34(8):932–938. doi: 10.1016/j.joen.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Colic M, Gazivoda D, Vucevic D, Vasilijic S, Rudolf R, Lukic A. Proinflammatory and immunoregulatory mechanisms in periapical lesions. Mol Immunol. 2009;47(1):101–113. doi: 10.1016/j.molimm.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 6.Fukada SY, Silva TA, Garlet GP, Rosa AL, da Silva JS, Cunha FQ. Factors involved in the T helper type 1 and type 2 cell commitment and osteoclast regulation in inflammatory apical diseases. Oral Microbiol Immunol. 2009;24(1):25–31. doi: 10.1111/j.1399-302X.2008.00469.x. [DOI] [PubMed] [Google Scholar]
- 7.Araujo-Pires AC, Biguetti CC, Repeke CE, Rodini Cde O, Campanelli AP, Trombone AP, et al. Mesenchymal stem cells as active prohealing and immunosuppressive agents in periapical environment: evidence from human and experimental periapical lesions. J Endod. 2014;40(10):1560–1565. doi: 10.1016/j.joen.2014.02.012. [DOI] [PubMed] [Google Scholar]
- 8.Araujo-Pires AC, Francisconi CF, Biguetti CC, Cavalla F, Aranha AM, Letra A, et al. Simultaneous analysis of T helper subsets (Th1, Th2, Th9, Th17, Th22, Tfh, Tr1 and Tregs) markers expression in periapical lesions reveals multiple cytokine clusters accountable for lesions activity and inactivity status. J Appl Oral Sci. 2014;22(4):336–346. doi: 10.1590/1678-775720140140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aranha AM, Repeke CE, Garlet TP, Vieira AE, Campanelli AP, Trombone AP, et al. Evidence supporting a protective role for th9 and th22 cytokines in human and experimental periapical lesions. J Endod. 2013;39(1):83–87. doi: 10.1016/j.joen.2012.10.015. [DOI] [PubMed] [Google Scholar]
- 10.Garlet GP, Horwat R, Ray HL, Jr, Garlet TP, Silveira EM, Campanelli AP, et al. Expression analysis of wound healing genes in human periapical granulomas of progressive and stable nature. J Endod. 2012;38(2):185–190. doi: 10.1016/j.joen.2011.09.011. [DOI] [PubMed] [Google Scholar]
- 11.AlShwaimi E, Purcell P, Kawai T, Sasaki H, Oukka M, Campos-Neto A, et al. Regulatory T cells in mouse periapical lesions. J Endod. 2009;35(9):1229–1233. doi: 10.1016/j.joen.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Colic M, Gazivoda D, Vucevic D, Majstorovic I, Vasilijic S, Rudolf R, et al. Regulatory T-cells in periapical lesions. J Dent Res. 2009;88(11):997–1002. doi: 10.1177/0022034509347090. [DOI] [PubMed] [Google Scholar]
- 13.Andrade AL, Nonaka CF, Gordon-Nunez MA, Freitas Rde A, Galvao HC. Immunoexpression of interleukin 17, transforming growth factor beta1, and forkhead box P3 in periapical granulomas, radicular cysts, and residual radicular cysts. J Endod. 2013;39(8):990–994. doi: 10.1016/j.joen.2013.04.028. [DOI] [PubMed] [Google Scholar]
- 14.Smigiel KS, Srivastava S, Stolley JM, Campbell DJ. Regulatory T-cell homeostasis: steady-state maintenance and modulation during inflammation. Immunol Rev. 2014;259(1):40–59. doi: 10.1111/imr.12170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sakaguchi S, Miyara M, Costantino CM, Hafler DA. FOXP3+ regulatory T cells in the human immune system. Nat Rev Immunol. 2010;10(7):490–500. doi: 10.1038/nri2785. [DOI] [PubMed] [Google Scholar]
- 16.Bruno KF, Silva JA, Silva TA, Batista AC, Alencar AH, Estrela C. Characterization of inflammatory cell infiltrate in human dental pulpitis. Int Endod J. 2010;43(11):1013–1021. doi: 10.1111/j.1365-2591.2010.01757.x. [DOI] [PubMed] [Google Scholar]
- 17.Peixoto RF, Pereira Jdos S, Nonaka CF, Silveira EJ, Miguel MC. Immunohistochemical analysis of FoxP3+ cells in periapical granulomas and radicular cysts. Arch Oral Biol. 2012;57(9):1159–1164. doi: 10.1016/j.archoralbio.2012.02.005. [DOI] [PubMed] [Google Scholar]
- 18.Marcal JR, Samuel RO, Fernandes D, de Araujo MS, Napimoga MH, Pereira SA, et al. T-helper cell type 17/regulatory T-cell immunoregulatory balance in human radicular cysts and periapical granulomas. J Endod. 2010;36(6):995–999. doi: 10.1016/j.joen.2010.03.020. [DOI] [PubMed] [Google Scholar]
- 19.Yang S, Zhu L, Xiao L, Shen Y, Wang L, Peng B, et al. Imbalance of interleukin-17+ T-cell and Foxp3+ regulatory T-cell dynamics in rat periapical lesions. J Endod. 2014;40(1):56–62. doi: 10.1016/j.joen.2013.09.033. [DOI] [PubMed] [Google Scholar]
- 20.Campos K, Franscisconi CF, Okehie V, de Souza LC, Trombone AP, Letra A, et al. FOXP3 DNA methylation levels as a potential biomarker in the development of periapical lesions. J Endod. 2015;41(2):212–218. doi: 10.1016/j.joen.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 21.He M, Song G, Yu Y, Jin Q, Bian Z. LPS-miR-34a-CCL22 axis contributes to regulatory T cell recruitment in periapical lesions. Biochem Biophys Res Commun. 2015;460(3):733–740. doi: 10.1016/j.bbrc.2015.03.098. [DOI] [PubMed] [Google Scholar]
- 22.Araujo-Pires AC, Vieira AE, Francisconi CF, Biguetti CC, Glowacki A, Yoshizawa S, et al. IL-4/CCL22/CCR4 axis controls regulatory T-cell migration that suppresses inflammatory bone loss in murine experimental periodontitis. J Bone Miner Res. 2015;30(3):412–422. doi: 10.1002/jbmr.2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Glowacki AJ, Yoshizawa S, Jhunjhunwala S, Vieira AE, Garlet GP, Sfeir C, et al. Prevention of inflammation-mediated bone loss in murine and canine periodontal disease via recruitment of regulatory lymphocytes. Proc Natl Acad Sci U S A. 2013;110(46):18525–18530. doi: 10.1073/pnas.1302829110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garlet GP, Cardoso CR, Campanelli AP, Martins W, Jr, Silva JS. Expression of suppressors of cytokine signaling in diseased periodontal tissues: a stop signal for disease progression? J Periodontal Res. 2006;41(6):580–584. doi: 10.1111/j.1600-0765.2006.00908.x. [DOI] [PubMed] [Google Scholar]
- 25.Roord ST, de Jager W, Boon L, Wulffraat N, Martens A, Prakken B, et al. Autologous bone marrow transplantation in autoimmune arthritis restores immune homeostasis through CD4+CD25+Foxp3+ regulatory T cells. Blood. 2008;111(10):5233–5241. doi: 10.1182/blood-2007-12-128488. [DOI] [PubMed] [Google Scholar]
- 26.Garlet GP, Cardoso CR, Mariano FS, Claudino M, de Assis GF, Campanelli AP, et al. Regulatory T cells attenuate experimental periodontitis progression in mice. J Clin Periodontol. 2010;37(7):591–600. doi: 10.1111/j.1600-051X.2010.01586.x. [DOI] [PubMed] [Google Scholar]
- 27.Yuan Q, Bromley SK, Means TK, Jones KJ, Hayashi F, Bhan AK, et al. CCR4-dependent regulatory T cell function in inflammatory bowel disease. J Exp Med. 2007;204(6):1327–1334. doi: 10.1084/jem.20062076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chow Z, Banerjee A, Hickey MJ. Controlling the fire - tissue-specific mechanisms of effector regulatory T-cell homing. Immunol Cell Biol. 2015;93(4):355–363. doi: 10.1038/icb.2014.117. [DOI] [PubMed] [Google Scholar]
- 29.Jhunjhunwala S, Raimondi G, Glowacki AJ, Hall SJ, Maskarinec D, Thorne SH, et al. Bioinspired controlled release of CCL22 recruits regulatory T cells in vivo. Adv Mater. 2012;24(35):4735–4738. doi: 10.1002/adma.201202513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yoshie O, Matsushima K. CCR4 and its ligands: from bench to bedside. Int Immunol. 2015;27(1):11–20. doi: 10.1093/intimm/dxu079. [DOI] [PubMed] [Google Scholar]
- 31.Li YQ, Liu FF, Zhang XM, Guo XJ, Ren MJ, Fu L. Tumor secretion of CCL22 activates intratumoral Treg infiltration and is independent prognostic predictor of breast cancer. PLoS One. 2013;8(10):e76379. doi: 10.1371/journal.pone.0076379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Montane J, Bischoff L, Soukhatcheva G, Dai DL, Hardenberg G, Levings MK, et al. Prevention of murine autoimmune diabetes by CCL22-mediated Treg recruitment to the pancreatic islets. J Clin Invest. 2011;121(8):3024–3028. doi: 10.1172/JCI43048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Flytlie HA, Hvid M, Lindgreen E, Kofod-Olsen E, Petersen EL, Jorgensen A, et al. Expression of MDC/CCL22 and its receptor CCR4 in rheumatoid arthritis, psoriatic arthritis and osteoarthritis. Cytokine. 2010;49(1):24–29. doi: 10.1016/j.cyto.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 34.Garlet GP, Sfeir CS, Little SR. Restoring host-microbe homeostasis via selective chemoattraction of Tregs. J Dent Res. 2014;93(9):834–839. doi: 10.1177/0022034514544300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Glowacki AJ, Gottardi R, Yoshizawa S, Cavalla F, Garlet GP, Sfeir C, et al. Strategies to direct the enrichment, expansion, and recruitment of regulatory cells for the treatment of disease. Ann Biomed Eng. 2015;43(3):593–602. doi: 10.1007/s10439-014-1125-2. [DOI] [PMC free article] [PubMed] [Google Scholar]