Abstract
In this study, we evaluated the associations of smoking and alcohol intake, both independently and collectively, with sodium intake in Korean men. Subjects (6340 men) were from the fifth Korean National Health Examination Survey (2010–2012). Smoking-related factors included smoking status, urinary cotinine level, and pack-years of smoking. Food intake was assessed using a 24-h recall. The odds of excessive sodium intake were estimated using survey logistic regression analysis. The smoking rate was 44.1%. The geometric mean of the urinary cotinine level was 0.05 µg/mL, and the median (min–max) pack-years of smoking was 13.2 (0–180). When adjusted for related factors, the odds (95% confidence interval) of excessive sodium intake were 1.54 (1.00, 2.37), 1.55 (1.23, 1.94), 1.44 (1.07, 1.95), and 1.37 (1.11, 1.68) times higher in the group exposed to smoking and drinking than in the group that never smoked nor drank, the group that never smoked and drank <5 times per month, the group that did not currently smoke and never drank, and the group that did not currently smoke or drink <5 times per month, respectively. There was an interaction effect between smoking and alcohol intake (p-interaction = 0.02). The results suggest that simultaneous exposure to smoking and alcohol intake is associated with increased odds of excessive sodium intake.
Keywords: smoking, alcohol intake, excessive sodium intake, KNHANES
1. Introduction
According to the Korean National Health Examination Survey (KNHANES), the smoking rate in Korea has consistently decreased among men aged ≥19 years, from 66.3% in 1998 to 42.1% in 2013 [1]. However, among OECD countries, the rate of smoking in Korea still ranks second, after Greece, for men aged ≥15 years [2]. Moreover, the starting age for smoking has decreased, from 14.1 years in 2005 to 13.9 years in 2010 and 13.7 years in 2014 [3].
Smoking has negative effects on psychological conditions such as depression [4] and increases the risk of arteriosclerosis, heart disease, and respiratory diseases such as asthma and chronic obstructive pulmonary disease [5]. It is the main cause of laryngeal, oral, pancreatic, and, in particular, lung cancers [6] and premature death [7]. It also affects taste perception [8,9], consistently dulling the perception of bitter [10] and sour tastes in current smokers [11], and smokers might have a greater preference for salty and sweet tastes than non-smokers [12].
Alcohol intake also adversely affects health, particularly when coupled with smoking, and smokers tend to have a higher alcohol intake than non-smokers [13,14]. A study based on the Korean Community Health Survey 2008 reported a negative association between a low-sodium diet and smoking and alcohol intake [15]. Furthermore, using the KNHANES 2010, the proportion of subjects with a sodium intake >4000 mg/day was reportedly higher in smoking and drinking groups [16].
Sodium is an essential element in the human body, and the daily dietary requirement of sodium is 1500 mg [17]. The daily sodium intake in Korea has steadily decreased, from 5861 mg in 2005 to 5597 mg in 2010 and 4659 mg in 2013. However, it remains more than twice that recommended by the World Health Organization (2000 mg) [1]. Excessive sodium intake is a major predisposing factor for increased blood pressure [18], stroke, and heart disease [19]. It also increases the risk of stomach cancer and is a risk factor for osteoporosis due to excretion of calcium from the bones [20].
Although the health effects of excessive sodium intake and unhealthy behaviors due to smoking and alcohol intake have been reported [15,16], few studies have evaluated the association between smoking and sodium intake in the general population and the combined effect, if any, of smoking and alcohol intake on sodium intake.
Therefore, this study aimed to evaluate the association of smoking and alcohol intake with excessive sodium intake among men using data from KNHANES V.
2. Materials and Methods
2.1. Study Subjects
Data were derived from the fifth KNHANES (2010–2012). The KNHANES is a series of national health surveys in Korea that use a stratified multistage probability sampling design to select a representative sampling of the Korean population. The KNHANES V health interview surveys were conducted through face-to-face interviews by trained interviewers at the homes of subjects. Informed consent was provided by each subject prior to inclusion in the study [21].
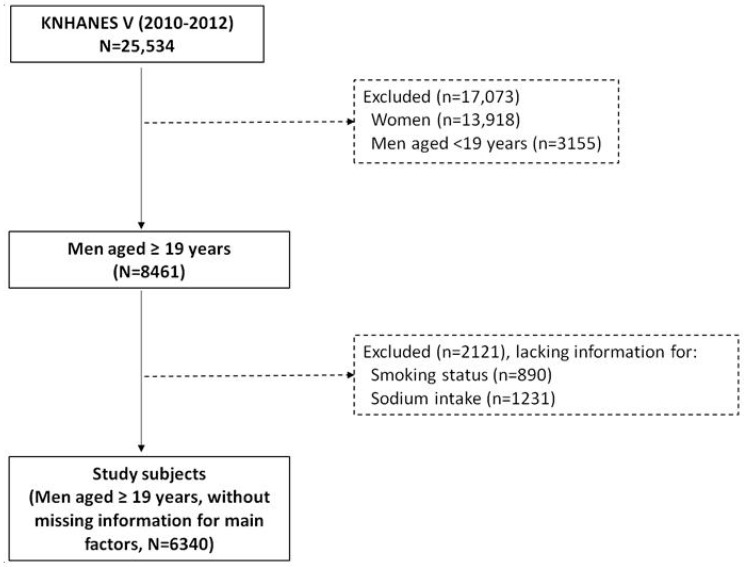
We included only men because they are the main consumer of tobacco, and women tend to underreport their smoking status due to social and cultural characteristics in Korea [22]. Initial candidates for this study were 25,534 subjects; of these, 13,918 women of any age and 3155 men aged <19 years were excluded. Further, 890 and 1231 subjects lacking information for smoking or sodium intake, respectively, were excluded. Finally, 6340 subjects were included (Figure 1).
Figure 1.
Selection process of study subjects, KNHANES V (2010–2012).
2.2. Smoking-Related Factors
Smoking-related factors included smoking status, urinary cotinine level, and pack-years of smoking. Smoking status was determined using the questionnaire and categorized as non-smoker, ex-smoker, or current smoker (including smoking often). Urinary cotinine levels were analyzed in 2010 and 2011 and measured using gas chromatography and mass spectrometry with a Perkin Elmer Clarus 600T (PerkinElmer, Turku, Finland), cotinine (Sigma, St. Louis, MO, USA), and diphenylamine (Aldrich, St. Louis, MO, USA) (threshold of detection, 0.25 ng/mL) [21]. We corrected for urine dilution by using urinary creatinine concentrations and calculated creatinine-standardized cotinine concentrations (vs. non-standardized) by dividing individual urinary cotinine concentrations by creatinine concentrations. Pack-years of smoking (Equation (1)) were calculated using information collected with the questionnaire and the following formula:
| (1) |
2.3. Sodium Intake
Food intake was assessed using a 24-h recall. Trained interviewers visited each subject’s house to collect food name, ingredients, and amount of intake during the previous 24 h. Data were converted to type and amount of food items in the form of units of food or nutrients, because the form of intake is a combination of foods. The reference food composition tables were from the seventh edition of the Korean National Rural Living Science Institute [23]. The Korea Health Industry Development Institute database was also used for some instant foods and imported foods [24]. Excessive sodium intake was defined as intake greater than the third quartile of sodium intake (>7392.52 mg/day).
2.4. Urinary Creatinine Level
Urinary creatinine level was analyzed in 2010 and 2012 and measured using a Creatinine-HR 1-Type Wako (WAKO, Osaka, Japan) with a Hitachi Automatic Analyzer 7600 (Hitachi, Tokyo, Japan) and colorimetry [21].
2.5. Confounding Factors
Age (19–34, 35–64, and ≥65 years), household income (low, lower-middle, upper-middle, and high), marital status (married; separated, widowed, or divorced; and never married), frequency of alcohol intake per month (none, 0–1, 2–4, and ≥5), and frequency of eating out (<1/month, 1–8/month, 3–6/week, and ≥1/day) were collected using the KNHANES questionnaire. Weight and height were measured by the KNHANES team to calculate body mass index (BMI), and we categorized participants as underweight (<18.5 kg/m2), normal weight (18.5–24.9 kg/m2), or overweight (≥25 kg/m2). When we analyzed non-standardized cotinine level, log transformed creatinine level was used for confounding factor. These confounding factors were previously used to estimate the risk of smoking and excessive sodium intake [15,16]. Among the socioeconomic status factors, household income and educational level were evaluated. Only household income was used in the final analyses because the associations between household income and smoking factors were greater than those with educational level.
2.6. Statistical Analysis
Survey chi-square tests were performed to evaluate the distribution of smoking status according to general characteristics. We used a weighted population sample to reflect the sampling method and response rate. Survey regression analysis was performed to assess the distribution of urinary cotinine levels, pack-years of smoking, and sodium intake according to general characteristics. Spearman correlation tests were performed to evaluate the correlations among the exposure variables. Odds ratios (ORs) and 95% confidence intervals (CIs) for excessive sodium intake were estimated using survey logistic regression analysis.
The p-trend was estimated using a continuous scale for each category. The p-interaction was estimated as the p-value of the interaction term between smoking status and alcohol intake. Missing values were excluded from the analyses with the general characteristics. Missing values were included in an “Unknown” category when variables with missing values were used as adjusting factors. All analyses were performed using SAS 9.4 [25], and significance was set at 0.05.
3. Results
3.1. Distribution of Smoking-Related Factors
Table 1 describes the distribution of smoking-related factors according to the general characteristics.
Table 1.
Distribution of smoking-related factors according to general characteristics among men aged ≥19 years in KNHANES V, 2010–2012.
| All | Current Smoker | Urinary Cotinine Level (μg/mL) | Pack-Years of Smoking | ||
|---|---|---|---|---|---|
| N | N | % w (SE) | GM a (95% CI) | Median (min–max) | |
| All | 6340 | 2450 | 44.1 (0.8) | 0.05 (0.04, 0.07) | 13.2 (0–180) |
| Age group (years) | |||||
| 19–34 | 1071 | 507 | 47.9 (1.6) | 0.06 (0.04, 0.08) | 2.4 (0–33) |
| 35–64 | 3545 | 1528 | 46.6 (1.0) | 0.06 (0.04, 0.07) | 15.8 (0–156) |
| ≥65 | 1724 | 415 | 25.1 (1.2) | 0.02 (0.01, 0.03) | 20.0 (0–180) |
| p-value | <0.0001 | 0.0003 | <0.0001 | ||
| Household income | |||||
| Low | 1202 | 439 | 42.7 (2.1) | 0.1 (0.06, 0.16) | 20.0 (0–156) |
| Lower middle | 1631 | 659 | 46.8 (1.7) | 0.07 (0.04, 0.10) | 13.5 (0–180) |
| Upper middle | 1744 | 681 | 44.9 (1.4) | 0.05 (0.03, 0.07) | 10.5 (0–129) |
| High | 1705 | 647 | 41.3 (1.6) | 0.04 (0.03, 0.06) | 10.3 (0–180) |
| Unknown | 58 | 58 | 47.8 (9.0) | 0.07 (0.00, 2.96) | 16.4 (0–85.5) |
| p-value | 0.01 | 0.03 | <0.0001 | ||
| Marital status | |||||
| Married | 5109 | 1868 | 42.1 (0.9) | 0.04 (0.03, 0.06) | 15.1 (0–180) |
| Separated, widowed, or divorced | 314 | 147 | 54.2 (3.6) | 0.21 (0.08, 0.53) | 23.9 (0–180) |
| Not married | 912 | 432 | 47.8 (1.8) | 0.07 (0.05, 0.10) | 2.0 (0–74) |
| Unknown | 5 | 3 | 58.3 (25.6) | - | 40.0 (12.9–86) |
| p-value | <0.0001 | 0.001 | <0.0001 | ||
| Frequency of alcohol intake (/month) | |||||
| None | 1098 | 244 | 24.1 (1.7) | 0.02 (0.01, 0.03) | 15.7 (0–147) |
| 0–1 | 1215 | 405 | 37.8 (1.8) | 0.03 (0.02, 0.05) | 8.3 (0–180) |
| 2–4 | 1666 | 675 | 45.1 (1.5) | 0.05 (0.03, 0.07) | 8.9 (0–150) |
| ≥5 | 2333 | 1116 | 54.2 (1.3) | 0.11 (0.08, 0.15) | 18.5 (0–180) |
| Unknown | 28 | 10 | 46.6 (8.8) | 0.02 (0.00, 0.19) | 7.5 (0–87.5) |
| p-value | <0.0001 | <0.0001 | <0.0001 | ||
| Body mass index (kg/m2) | |||||
| <18.5 | 192 | 92 | 51.4 (4.8) | 0.04 (0.01, 0.32) | 17.0 (0–156) |
| 18.5 to <25 | 3940 | 1538 | 43.8 (1.0) | 0.05 (0.04, 0.07) | 12.8 (0–180) |
| ≥25 | 2182 | 807 | 44.1 (1.3) | 0.06 (0.04, 0.08) | 14.0 (0–180) |
| Unknown | 26 | 13 | 44.7 (11.5) | 0.01 (0, 6203.2) | 10.5 (0–45) |
| p-value | 0.14 | 0.93 | 0.006 | ||
| Frequency of eating out | |||||
| <1/month | 646 | 214 | 35.4 (2.5) | 0.04 (0.01, 0.11) | 24.8 (0–156) |
| 1–8/month | 2131 | 712 | 39.4 (1.5) | 0.06 (0.04, 0.08) | 17.5 (0–180) |
| 3–6/week | 1615 | 633 | 45.4 (1.5) | 0.05 (0.03, 0.07) | 10.0 (0–180) |
| ≥1/day | 1946 | 890 | 48.5 (1.4) | 0.06 (0.04, 0.08) | 10.0 (0–140) |
| Unknown | 2 | 1 | 42.6 (35.0) | - | 8.5 (0.5–16.5) |
| p-value | <0.0001 | 0.70 | <0.0001 | ||
| Correlation coefficient with urinary cotinine level (p-value) | 0.63 (<0.001) | - | 0.27 (<0.001) | ||
a SE: standard error; GM: geometric mean; CI: confidence interval; p-value estimated using survey chi-square tests or survey regression analyses.
The smoking rate was 44.1%. The geometric mean (95% CI) of the urinary cotinine level was 0.05 (0.04, 0.07) µg/mL, and the median (min–max) pack-years of smoking was 13.2 (0–180). Smoking rates and urinary cotinine levels were higher with younger age and more frequent alcohol intake. The factors associated with smoking rate and urinary cotinine level, in increasing order of effect, were lower-middle income, marital separation, and obesity. Pack-years of smoking were higher with older age, rarely eating out, and low income. Marital separation and underweight were related with pack-years of smoking. The correlation between smoking status and urinary cotinine level (ρ = 0.63, p < 0.001) was higher than that between pack-years of smoking and urinary cotinine level (ρ = 0.27, p < 0.001).
3.2. Distribution of Sodium Intake
Table 2 describes the distribution of sodium intake according to the general characteristics. The median sodium intake was significantly different across the distributions of all of the characteristics. Sodium intake was significantly lower in the older and marital separation groups. It was higher with increasing income, frequency of alcohol intake, BMI, and frequency of eating out.
Table 2.
Distribution of sodium intake (mg/day) according to general characteristics among men aged ≥19 years in KNHANES V, 2010–2012.
| All | Sodium Intake (mg/day) | |
|---|---|---|
| N | GM a (95% CI) | |
| All | 6340 | 5133.2 (5034.5, 5233.9) |
| Age group (years) | ||
| 19–34 | 1071 | 5170.7 (4967.5, 5382.2) |
| 35–64 | 3545 | 5432.3 (5310.6, 5556.8) |
| ≥65 | 1724 | 3955.6 (3802.2, 4115.1) |
| p-value | <0.0001 | |
| Household income | ||
| Low | 1202 | 4253.6 (4047.6, 4470.1) |
| Lower middle | 1631 | 5050.2 (4868.3, 5238.8) |
| Upper middle | 1744 | 5409.6 (5242.9, 5581.7) |
| High | 1705 | 5442.4 (5232.8, 5660.4) |
| Unknown | 58 | 4707.9 (4111.7, 5390.4) |
| p-value | <0.0001 | |
| Marital status | ||
| Married | 5109 | 5239.5 (5132.6, 5348.6) |
| Separated, widowed, or divorced | 314 | 4743.3 (4346.6, 5176.3) |
| Not married | 912 | 4920.9 (4712.2, 5138.9) |
| Unknown | 5 | - |
| p-value | 0.01 | |
| Frequency of alcohol intake (/month) | ||
| None | 1098 | 4547.9 (4324.1, 4783.4) |
| 0–1 | 1215 | 4900.7 (4699.0, 5111.1) |
| 2–4 | 1666 | 5195.0 (5013.7, 5382.7) |
| ≥5 | 2333 | 5450.9 (5297.4, 5609.0) |
| Unknown | 28 | 5797.3 (4817.5, 6976.4) |
| p-value | <0.0001 | |
| Body mass index (kg/m2) | ||
| <18.5 | 192 | 4540.5 (4119.9, 5004.0) |
| 18.5 to <25 | 3940 | 4952.6 (4835.7, 5072.2) |
| ≥25 | 2182 | 5510.2 (5342.6, 5683.1) |
| Unknown | 26 | 4834.9 (3718.6, 6286.3) |
| p-value | <0.0001 | |
| Frequency of eating out | ||
| <1/month | 646 | 3906.3 (3622.5, 4212.3) |
| 1–8 month | 2131 | 4636.1 (4474.7, 4803.2) |
| 3–6/week | 1615 | 5397.2 (5213.6, 5587.1) |
| ≥1/day | 1946 | 5637.5 (5463.5, 5817.1) |
| Unknown | 2 | 6665.4 (29.7, 1,498,256.3) |
| p-value | <0.0001 |
GM: geometric mean; CI: confidence interval; p-value estimated using survey regression analysis.
3.3. Odds of Excessive Sodium Intake According to Smoking-Related Factors
Table 3 describes the odds for excessive sodium intake according to the smoking-related factors. The odds were higher for current smokers and the group with the highest cotinine levels, but this was not statistically significant when adjusted for related factors.
Table 3.
Odds ratios (ORs) and 95% confidence intervals (CIs) for excessive sodium intake according to smoking-related factors among men aged ≥19 years in KNHANES V, 2010–2012.
| Smoking-Related Factors | Excessive Sodium Intake (>7392.52 mg/day) a | |||
|---|---|---|---|---|
| Crude Model | Adjusted Model b | |||
| N | Cases | OR (95% CI) | OR (95% CI) | |
| Smoking status | ||||
| Non-smoker | 1176 | 264 | 1.00 (ref) | 1.00 (ref) |
| Ex-smoker | 2714 | 666 | 1.21 (1.00, 1.47) | 1.16 (0.94, 1.43) |
| Current smoker | 2450 | 655 | 1.33 (1.10, 1.61) | 1.17 (0.95, 1.43) |
| p-trend | 0.01 | 0.22 | ||
| Urinary cotinine level (μg/mL) | ||||
| Q1 (0.009, 3.07) | 387 | 112 | 1.00 (ref) | 1.00 (ref) c |
| Q2 (3.07, 14.5) | 387 | 104 | 0.82 (0.56, 1.21) | 0.77 (0.52, 1.14) |
| Q3 (14.5, 1133.38) | 388 | 110 | 0.99 (0.70, 1.41) | 0.96 (0.66, 1.40) |
| Q4 (1133.38, 5800.17) | 387 | 137 | 1.31 (0.93, 1.84) | 1.29 (0.89, 1.86) |
| p-trend | 0.05 | 0.08 | ||
| Urinary cotinine level corrected by creatinine level (μg/g Cr) | ||||
| Q1 (0.00002, 0.02) | 387 | 106 | 1.00 (ref) | 1.00 (ref) |
| Q2 (0.02, 0.11) | 387 | 108 | 0.85 (0.57, 1.27) | 0.83 (0.55, 1.26) |
| Q3 (0.11, 6.63) | 388 | 112 | 1.10 (0.76, 1.57) | 1.07 (0.73, 1.56) |
| Q4 (6.63, 63.78) | 387 | 137 | 1.34 (0.94, 1.90) | 1.26 (0.87, 1.83) |
| p-trend | 0.04 | 0.11 | ||
| Pack-years of smoking | ||||
| Q1 (0, 1.3) | 1531 | 350 | 1.00 (ref) | 1.00 (ref) |
| Q2 (1.3, 13) | 1517 | 400 | 1.28 (1.05, 1.56) | 1.15 (0.94, 1.41) |
| Q3 (13, 28) | 1539 | 423 | 1.29 (1.06, 1.58) | 1.10 (0.88, 1.38) |
| Q4 (28, 180) | 1511 | 342 | 1.09 (0.89, 1.32) | 1.08 (0.86, 1.36) |
| p-trend | 0.27 | 0.53 | ||
ORs and 95% CIs were estimated using survey logistic regression models. a Third quartile of sodium intake (mg/day) in KNHANES V; b Adjusted for age, household income, marital status, body mass index, frequency of alcohol intake, and frequency of eating out; c Additionally adjusted for log-transformed creatinine level. The p-trend was estimated using the continuous scale of each category in the same model.
3.4. Excessive Sodium Intake According to Alcohol Intake
Table 4 shows the odds for excessive sodium intake according to the frequency of alcohol intake. When adjusted for related factors, the odds of excessive sodium intake were 1.49 times (95% CI: 1.14, 1.95) higher in the group with an alcohol intake >5 times per month than in the non-alcohol intake group, and the OR increased with increasing frequency of alcohol intake per month (p-trend = 0.003).
Table 4.
Odds ratios (ORs) and 95% confidence intervals (CIs) for excessive sodium intake according to the frequency of alcohol intake per month among men aged ≥19 years in KNHANES V, 2010–2012.
| Excessive Sodium Intake (>7392.52 mg/day a) | ||||
|---|---|---|---|---|
| N | Cases | Crude model | Adjusted model b | |
| OR (95% CI) | OR (95% CI) | |||
| Frequency of alcohol intake (/month) | ||||
| None | 1098 | 190 | 1.00 (ref) | 1.00 (ref) |
| 0–1 | 1215 | 287 | 1.43 (1.08, 1.90) | 1.25 (0.93, 1.67) |
| 2–4 | 1666 | 425 | 1.43 (1.11, 1.85) | 1.17 (0.90, 1.52) |
| ≥5 | 2333 | 675 | 1.85 (1.43, 2.38) | 1.49 (1.14, 1.95) |
| p-trend | <0.0001 | 0.003 | ||
ORs and 95% CIs were estimated using survey logistic regression models. a Third quartile of sodium intake (mg/day) in KNHANES V; b Adjusted for age, household income, marital status, body mass index, frequency of eating out, and smoking status. The p-trend was estimated using the continuous scale of each category in the same model.
3.5. Excessive Sodium Intake According to Combined Smoking and Alcohol Intake
Table 5 shows the odds for excessive sodium intake according to combined smoking status and current alcohol intake. When adjusted for related factors, the odds (95% CI) for excessive sodium intake in the group exposed to both smoking and drinking were 1.54 times (1.00, 2.37), 1.55 times (1.23, 1.94), 1.44 times (1.07, 1.95), and 1.37 times (1.11, 1.68) higher than in the group that never smoked nor drank, the group that never smoked and drank <5 times per month, the group that did not currently smoke and never drank, and the group that did not currently smoke and drank <5 times per month, respectively. In addition, there was an interaction effect between smoking experience and alcohol intake (p-interaction = 0.02).
Table 5.
Odds ratios (ORs) and 95% confidence intervals (CIs) for excessive sodium intake according to smoking status and alcohol intake among men aged ≥19 years in KNHANES V, 2010–2012.
| Risk Group of Excessive Sodium Intake (>7392.52 mg/day) a | |||||
|---|---|---|---|---|---|
| N | Case | Crude Model | Adjusted Model b | ||
| OR (95% CI) | OR (95% CI) | ||||
| Smoking experience c | Current alcohol intake | ||||
| No | No | 279 | 46 | 1.00 (ref) | 1.00 (ref) |
| No | Yes | 893 | 217 | 1.39 (0.88, 2.18) | 1.27 (0.80, 2.02) |
| Yes | No | 819 | 144 | 1.08 (0.67, 1.72) | 1.15 (0.71, 1.86) |
| Yes | Yes | 4321 | 1170 | 1.76 (1.16, 2.67) | 1.54 (1.00, 2.37) |
| p-interaction | 0.0005 | 0.02 | |||
| Smoking experience c | Frequency of alcohol intake (≥5/month) | ||||
| No | No | 912 | 190 | 1.00 (ref) | 1.00 (ref) |
| No | Yes | 260 | 73 | 1.45 (0.98, 2.15) | 1.40 (0.94, 2.08) |
| Yes | No | 3067 | 712 | 1.23 (0.99, 1.52) | 1.22 (0.97, 1.53) |
| Yes | Yes | 2073 | 602 | 1.64 (1.33, 2.02) | 1.55 (1.23, 1.94) |
| p-interaction | 0.11 | 0.22 | |||
| Current smoker | Current alcohol intake | ||||
| No | No | 854 | 145 | 1.00 (ref) | 1.00 (ref) |
| No | Yes | 3018 | 777 | 1.53 (1.16, 2.02) | 1.30 (0.98, 1.74) |
| Yes | No | 244 | 45 | 1.07 (0.66, 1.74) | 1.00 (0.61, 1.63) |
| Yes | Yes | 2196 | 610 | 1.75 (1.31, 2.33) | 1.44 (1.07, 1.95) |
| p-interaction | 0.48 | 0.50 | |||
| Current smoker | Frequency of alcohol intake (≥5/month) | ||||
| No | No | 2655 | 567 | 1.00 (ref) | 1.00 (ref) |
| No | Yes | 1217 | 355 | 1.54 (1.26, 1.87) | 1.46 (1.20, 1.79) |
| Yes | No | 1324 | 335 | 1.26 (1.04, 1.53) | 1.19 (0.98, 1.46) |
| Yes | Yes | 1116 | 320 | 1.5 (1.23, 1.83) | 1.37 (1.11, 1.68) |
| p-interaction | 0.02 | 0.05 | |||
ORs and 95% CIs were estimated using survey logistic regression models. a Third quartile of sodium intake (mg/day) in KNHANES V; b Adjusted for age, household income, marital status, body mass index, and frequency of eating out; c Smoking experience: no (never smoker) and yes (ex-smoker and current smoker). The p-interaction was estimated as the p-value of the interaction term between smoking status and alcohol intake, adjusted for the same confounding factors.
4. Discussion
We found that the combination of smoking and alcohol intake was associated with increased odds of excessive sodium intake. The results of the present study corroborate the findings of previous studies [26,27]; pack-years of smoking were higher in older men, but smoking rate and urinary cotinine levels were lower with increasing age.
In the present study, smoking was not significantly associated with excessive sodium intake, which is similar to the results of a previous study using the fourth KNHANES in which sodium intake was much higher in light smokers than in non-smokers, but this was not significant [28]. However, our results differ from those of other studies [12,16]. Among middle-aged Korean workers, the proportion of current smokers with a sodium intake >4000 mg was higher than the proportion of non-smokers and past smokers [16]. Furthermore, in another study with subjects aged ≥18 years, the preference for saltier food was 1.5–2.3 times higher in smokers than in non-smokers [12].
In studies using a diet preference, smoking was associated with sodium intake [12,28]. However, in studies using a 24-h recall, the results were not significantly associated [28], as in the present study. Thus, the method of measuring sodium intake and the sample might yield varying results. In the present study, excessive sodium intake was significantly more likely with increasing frequency of alcohol intake, supporting the findings of previous studies [16,29]. The proportion of subjects with a sodium intake >4000 mg was higher in those who consumed alcohol [16], and a positive correlation between alcohol intake and sodium intake was reported among subjects aged ≥ 65 years [29]. Alcohol drinkers prefer saltier food 2.57–2.92 times more than non-drinkers [12], and the preference for salty tastes increases with increased smoking, alcohol intake, and obesity [30,31]. Therefore, unhealthy lifestyle factors such as smoking and alcohol intake might affect the preference for salt. Alcohol intake might be a consistent factor for excessive sodium intake because most Korean people eat salty foods while drinking alcohol, and these foods typically have a higher salt content than a usual meal [32].
In the present study, the sodium intake was 1.54 times higher in the group of subjects with both a current or previous history of smoking and current alcohol intake than in the group of subjects who were both never-smokers and non-drinkers (p-interaction = 0.02). Similarly, compared with non-smokers and non-drinkers, the preference for low-salt food decreased in the following order in a previous study: smoking only group, alcohol only group, and both smoking and alcohol group [15]. Exposure to both smoking and alcohol has a more serious impact on health than smoking or alcohol alone [33]. The correlations between current smoking and current drinking and between current smoking and hazardous drinking are statistically significant [34]. In addition, smokers tend to consume more alcohol than non-smokers [13,14]; drinking might lead to greater smoking, while smoking might encourage increased drinking. This bi-directional relationship might explain a high sodium intake with high alcohol intake [35].
Owing to the self-reported nature of the questionnaire, the present results might have underestimated smoking and alcohol consumption while overestimating other variables as a result of social desirability. In addition, because smoking dulls taste perception, a causal relationship with excessive sodium intake could not be explained in an earlier cross-sectional study [36]. However, a 24-h recall might be a more appropriate method than a food frequency questionnaire to consider ordinal associations, despite the inability of a 24-h recall to consider dietary patterns for sodium intake [37].
Despite these limitations, the 3-year nationwide KNHANES survey was conducted with representative subjects of the population; therefore, the results likely represent the general population. The reliability of the results is also high because the quality management was conducted in accordance with standard performance guidelines. Moreover, the use of three smoking factors increases the validity of the results.
5. Conclusions
Excessive sodium intake was higher with smoking experience and alcohol intake in Korean men, owing to a simultaneous effect of smoking and alcohol consumption. The main cause and route of excessive sodium intake identified in this study could be utilized as the primary basis of future measures for dietary sodium reduction policies and to identify high-risk groups.
Author Contributions
Kyung-Hwa Choi analyzed the data and wrote the paper. Myung-Sook Park and Jung Ae Kim wrote the manuscript and interpreted the results. Ji-Ae Lim provided critical and constructive comments.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Korea Centers for Disease Control and Prevention . Statistics of Health Behavior and Chronic Disease. Ministry of Health & Welfare; Cheongju, Chungbuk, Korea: 2013. [Google Scholar]
- 2.OECD Stat. Tobacco Consumption, % of Males Aged 15+ Who Are Daily Smokers. 2014. [(accessed on 30 August 2015)]. Available online: http://www.oecd-ilibrary.org/social-issues-migration-health/data/oecd-health-statistics/oecd-health-data-non-medical-determinants-of-health_data-00546-en;jsessionid=3eg7tl219a60a.x-oecd-live-02?isPartOf=/content/datacollection/health-data-en.
- 3.Korea Centers for Disease Control and Prevention . Statistics of the Tenth Korea Youth Risk Behavior Web-Based Survey. Korea Centers for Disease Control and Prevention; Cheongju, Chungbuk, Korea: 2014. [Google Scholar]
- 4.Chu J.E., Lee H., Yoon C.H., Cho H.-I., Hwang J.-Y., Park Y.J. Relationships between depressed mood and life style patterns in Koreans aged 40 years. J. Korean Soc. Food Sci. Nutr. 2014;43:772–783. doi: 10.3746/jkfn.2014.43.5.772. [DOI] [Google Scholar]
- 5.Tamimi A., Serdarevic D., Hanania N.A. The effects of cigarette smoke on airway inflammation in asthma and COPD: Therapeutic implications. Respir. Med. 2012;106:319–328. doi: 10.1016/j.rmed.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 6.Kushi L.H., Doyle C., McCullough M., Rock C.L., Demark-Wahnefried W., Bandera E.V., Gapstur S., Patel A.V., Andrews K., Gansler T. American cancer society guidelines on nutrition and physical activity for cancer prevention: Reducing the risk of cancer with healthy food choices and physical activity. CA Cancer J. Clin. 2012;62:30–67. doi: 10.3322/caac.20140. [DOI] [PubMed] [Google Scholar]
- 7.Muezzinler A., Mons U., Gellert C., Schottker B., Jansen E., Kee F., O’Doherty M.G., Kuulasmaa K., Freedman N.D., Abnet C.C., et al. Smoking and all-cause mortality in older adults: Results from the chances consortium. Am. J. Prev. Med. 2015;49:e53–e63. doi: 10.1016/j.amepre.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 8.Sato K., Endo S., Tomita H. Sensitivity of three loci on the tongue and soft palate to four basic tastes in smokers and non-smokers. Acta Otolaryngol. Suppl. 2002;122:74–82. doi: 10.1080/00016480260046445. [DOI] [PubMed] [Google Scholar]
- 9.Vennemann M.M., Hummel T., Berger K. The association between smoking and smell and taste impairment in the general population. J. Neurol. 2008;255:1121–1126. doi: 10.1007/s00415-008-0807-9. [DOI] [PubMed] [Google Scholar]
- 10.Jacob N., Golmard J.-L., Berlin I. Differential perception of caffeine bitter taste depending on smoking status. Chemosens. Percept. 2014;7:47–55. doi: 10.1007/s12078-014-9164-5. [DOI] [Google Scholar]
- 11.Fischer M.E., Cruickshanks K.J., Schubert C.R., Pinto A., Klein B.E., Klein R., Nieto F.J., Pankow J.S., Huang G.H., Snyder D.J. Taste intensity in the beaver dam offspring study. Laryngoscope. 2013;123:1399–1404. doi: 10.1002/lary.23894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lampure A., Schlich P., Deglaire A., Castetbon K., Peneau S., Hercberg S., Mejean C. Sociodemographic, psychological, and lifestyle characteristics are associated with a liking for salty and sweet tastes in french adults. J. Nutr. 2015;145:587–594. doi: 10.3945/jn.114.201269. [DOI] [PubMed] [Google Scholar]
- 13.John U., Meyer C., Rumpf H.J., Schumann A., Thyrian J.R., Hapke U. Strength of the relationship between tobacco smoking, nicotine dependence and the severity of alcohol dependence syndrome criteria in a population-based sample. Alcohol Alcohol. 2003;38:606–612. doi: 10.1093/alcalc/agg122. [DOI] [PubMed] [Google Scholar]
- 14.Joe K.H., Kim D.J. The comorbidity of alcohol dependence and nicotine dependence. Korean J. Psychopharmacol. 2008;19:85–93. [Google Scholar]
- 15.Chun I., Park J., Han M.-A., Choi S., Ryu S.-Y. The association between smoking, alcohol intake, and low-salt diet: Results from the 2008 community health survey. J. Korean Diet. Assoc. 2013;19:223–235. doi: 10.14373/JKDA.2013.19.3.223. [DOI] [Google Scholar]
- 16.Kim M.-G., Kim K.-Y., Nam H.-M., Hong N.-S., Lee Y.-M. The relationship between lifestyle and sodium intake in Korean middle-aged workers. J. Korea Academ. Ind. Coop. Soc. 2014;15:2923–2929. doi: 10.5762/KAIS.2014.15.5.2923. [DOI] [Google Scholar]
- 17.Ministry of Health and Welfare . Guide Book of Korean Nutrient Intake Standard 2010. Ministry of Health and Welfare; Seoul, Korea: 2013. [Google Scholar]
- 18.Freedman D.A., Petitti D.B. Salt and blood pressure. Conventional wisdom reconsidered. Eval. Rev. 2001;25:267–287. doi: 10.1177/0193841X0102500301. [DOI] [PubMed] [Google Scholar]
- 19.He F.J., Li J., Macgregor G.A. Effect of longer-term modest salt reduction on blood pressure. Cochrane Database Syst. Rev. 2013;4 doi: 10.1002/14651858.CD004937. [DOI] [PubMed] [Google Scholar]
- 20.Park D.I., Choi-Kwon S., Han K. Health behaviors of Korean female nursing students in relation to obesity and osteoporosis. Nurs. Outlook. 2015;63:504–511. doi: 10.1016/j.outlook.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 21.Korea Centers For Disease Control and Prevention . Guide for Survey of Korean National Health & Nutrition Examination Survey, 2010–2012. Korea Centers for Disease Control and Prevention; Cheongju, Chungbuk, Korea: 2014. [Google Scholar]
- 22.Jung-Choi K.H., Khang Y.H., Cho H.J. Hidden female smokers in Asia: A comparison of self-reported with cotinine-verified smoking prevalence rates in representative national data from an Asian population. Tob. Control. 2012;21:536–542. doi: 10.1136/tobaccocontrol-2011-050012. [DOI] [PubMed] [Google Scholar]
- 23.National Rural Living Science Institute . The 7th Food Composition Table in Korea in 1996, 2001, 2006. National Rural Living Science Institute; Suwon, Korea: 2006. [Google Scholar]
- 24.Ministry of Health and Welfare . Development of Recipe Database for Korea Health and Nutrition Examination. Korea Health Industry Development Institute; Cheongju, Korea: 1998. [Google Scholar]
- 25.SAS proprietary software 9.4. SAS Institute; Cary, NC, USA: 2015. [Google Scholar]
- 26.Rostron B.L., Chang C.M., Pechacek T.F. Estimation of cigarette smoking-attributable morbidity in the United States. JAMA Intern. Med. 2014;174:1922–1928. doi: 10.1001/jamainternmed.2014.5219. [DOI] [PubMed] [Google Scholar]
- 27.Cho S., Shin A., Park S.K., Shin H.R., Chang S.H., Yoo K.Y. Alcohol drinking, cigarette smoking and risk of colorectal cancer in the Korean multi-center cancer cohort. J. Cancer Prev. 2015;20:147–152. doi: 10.15430/JCP.2015.20.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yeon J.-Y., Kim E.-Y., Lee E.-J., Bae Y.-J. Relationship among pack-years of smoking, metabolic biomarkers, and diet quality in male adults: From the Korean national health and nutrition examination surveys, 2007–2009. J. East Asian Soc. Diet. Life. 2012;22:175–189. [Google Scholar]
- 29.Jang J.-Y., Kim M.-J., Han J.-S. A study on food frequency, dietary habits and nutrition knowledge of the elderly who intake high sodium. J. Korean Soc. Food Sci. Nutr. 2009;38:1362–1372. doi: 10.3746/jkfn.2009.38.10.1362. [DOI] [Google Scholar]
- 30.Lampure A., Deglaire A., Schlich P., Castetbon K., Peneau S., Hercberg S., Mejean C. Liking for fat is associated with sociodemographic, psychological, lifestyle and health characteristics. Br. J. Nutr. 2014;112:1353–1363. doi: 10.1017/S0007114514002050. [DOI] [PubMed] [Google Scholar]
- 31.Mejean C., Macouillard P., Castetbon K., Kesse-Guyot E., Hercberg S. Socio-economic, demographic, lifestyle and health characteristics associated with consumption of fatty-sweetened and fatty-salted foods in middle-aged French adults. Br. J. Nutr. 2011;105:776–786. doi: 10.1017/S0007114510004174. [DOI] [PubMed] [Google Scholar]
- 32.Lee S.H., Kim W.Y. Relationship of habitual alcohol consumption to the nutritional status in middle aged men. Korean J. Nutr. 1991;24:58–65. [Google Scholar]
- 33.Talamini R., Bosetti C., La Vecchia C., Dal Maso L., Levi F., Bidoli E., Negri E., Pasche C., Vaccarella S., Barzan L., et al. Combined effect of tobacco and alcohol on laryngeal cancer risk: A case-control study. Cancer Causes Control. 2002;13:957–964. doi: 10.1023/A:1021944123914. [DOI] [PubMed] [Google Scholar]
- 34.Katulanda P., Ranasinghe C., Rathnapala A., Karunaratne N., Sheriff R., Matthews D. Prevalence, patterns and correlates of alcohol consumption and its’ association with tobacco smoking among sri lankan adults: A cross-sectional study. BMC Public Health. 2014;14 doi: 10.1186/1471-2458-14-612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dierker L., Lloyd-Richardson E., Stolar M., Flay B., Tiffany S., Collins L., Bailey S., Nichter M., Nichter M., Clayton R. The proximal association between smoking and alcohol use among first year college students. Drug Alcohol Depend. 2006;81:1–9. doi: 10.1016/j.drugalcdep.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 36.Jeong J.R., Kim S., Jo S.R., Joh J.Y., Kim Y.P. Health behaviors of breast cancer survivors with hypertension: A propensity analysis of KNHANES III-V (2005–2012) PLoS One. 2015;10 doi: 10.1371/journal.pone.0127346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim D.W., Oh S.Y., Kwon S.O., Kim J. Comparison of validity of food group intake by food frequency questionnaire between pre- and post-adjustment estimates derived from 2-day 24-hour recalls in combination with the probability of consumption. Asian Pac. J. Cancer Prev. 2012;13:2655–2661. doi: 10.7314/APJCP.2012.13.6.2655. [DOI] [PubMed] [Google Scholar]