Abstract
Traditional Chinese medicines (TCMs) have attracted extensive interest throughout the world due to their long history of health protection and disease control, and the internalization of TCM preparations or patented drugs has been considered a wind vane in the process of TCM modernization. However, multi-target effects, caused by multiple components in TCMs, hinder not only the construction of the quality evaluation system (bioavailability), but also the application of pharmaceutical technologies, which results in the poor efficacy in clinical practice. This review describes the methods in the literature as well as in our thoughts about how to identify the marker components, establish the evaluation system of bioavailability, and improve the bioavailability in TCM preparations. We expect that the current study will be positive and informative.
Keywords: bio-pharmaceutics of TCMs preparations, active constitutes identification, evaluation system of bioavailability, pharmaceutical technologies, absorption enhancer
1. Introduction
Traditional Chinese medicines (TCMs), utilized in the prevention and treatment of various diseases for thousands of years in China, have been gradually accepted and employed in other countries. TCM preparations or patented drugs, defined by the utilization of herbs, animals, and minerals, with their respective dosages in accordance with the guidance of Chinese medicine theory and the rule of “King, Vassal, Assistant and Delivery servant”, have different dosage forms, such as capsules, tablets, pills, powders, oral liquids, etc. [1]. It was reported that the Chinese export of herbal medicines and extracts was significantly higher than that of preparations in the recent years. As shown in 2014, the export of herbal medicines and extracts was worth 2.95 billion dollars, but that of preparations was little, only 250 million dollars [2], which was mainly due to the unsound quality evaluation system (bioavailability) and poor efficacy in clinical practice. For example, Shuang-Huang-Lian oral liquid, a well-known TCM preparation composited of Flos Lonicerae Japonicae, Fructus Forsythiae, and Radix Scutellariae, is usually used as the treatment for acute upper respiratory tract infection caused by bacteria and viruses, but its clinical efficacy was unstable and far lower than that of injection [3]. It was found that multi-target effects, caused by multiple components in TCMs, hindered not only the construction of the evaluation system of bioavailability, but also the formulation, designation, and technologies application. How to establish a quantifiable evaluation system of bioavailability and find suitable pharmaceutical technologies to improve the bioavailability was not only a basic scientific problem of bio-pharmaceutics for TCM preparations, but it was also the key factor in modernizing TCMs.
The following essential problems that refer to the evaluation system of bioavailability construction and pharmaceutical technology applications for TCM preparations exist. Firstly, the network pharmacological effects and the complex structure-effect and dose-effect relationships in TCMs contributed to difficulty in identifying the effective components; Secondly, biological active and pharmacokinetic (absorption, distribution, metabolism, and excretion) diversity of effective constituents resulted in obstacles for setting up weight coefficients for integrating bioavailability; Thirdly, pharmaceutical technologies were hardly applied for TCM preparations due to their complicated physico-chemical properties for both active ingredients and associated constituents.
Therefore, the current problems about how to identify the active components promptly; how to establish a reasonable mathematics model to calculate the weight coefficient to integrate bioavailability; and how to improve the integral bioavailability using related pharmaceutical technologies in TCM preparations need to be further investigated.
2. Identification of Active Compounds in Traditional Chinese Medicines (TCMs)
2.1. Classic Separation and Analysis
The classic separation and analysis model was performed to identify the active components according to the procedures of extraction, separation, purification, characterization, pharmacological tests, etc., and it was applied to new Chinese herbal monomer or Chinese herbal extract development. For example, artemisinin isolated from the plant Artemisia annua, sweet wormwood, and its derivatives possess the most rapid actions against Plasmodium falciparum malaria [4]. Digoxin was a purified cardiac glycoside, extracted from Digitalis lanata, and was occasionally used to treat various heart diseases, namely atrial fibrillation and atrial flutter [5]. Morphine, a pain medication of the opiate type extracted from papaver somniferum L., can decrease feelings of pain through acting directly on the central nervous system (CNS) [6]. Paclitaxel, extracted from the Yew tree, is an anti-cancer drug. It was the first-line treatment for cancers of the breast, colon, lung, etc., and the second-line treatment for AIDS-related Kaposi’s sarcoma [7]. The chemotherapy agent (vincristine), extracted from Catharanthus roseus, was utilized as the treatment of leukemias, lymphomas, etc. [8]. The total lactones, a Chinese herbal extract from the Ginkgo leaf, contained mainly ginkgo lactone A, ginkgo lactone B, ginkgo lactone C, and ginkgo seed lactone, which were prepared as a medicine for preventing or treating deafness and tinnitus [9]. The tea polyphenols, included catechins, theaflavins, tannins, and flavonoids, can prevent coronary heart disease and cancer [10].
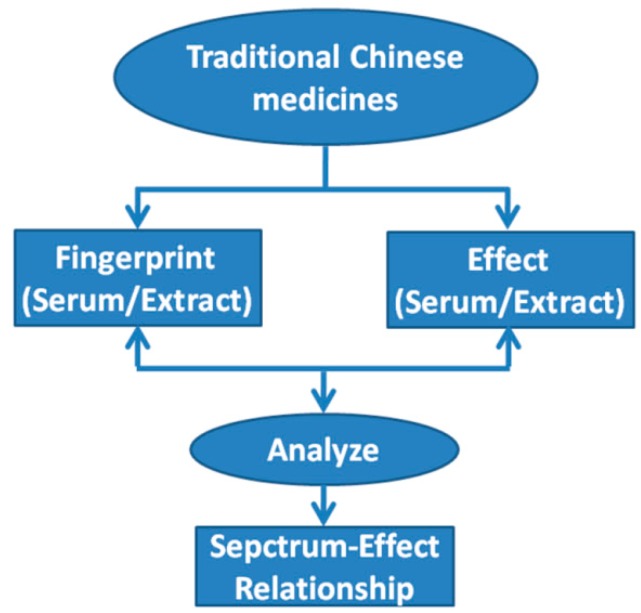
2.2. Spectrum-Effect Relationships
The spectrum-effect relationship, put forward firstly by Li et al., 2002 [11], is an effective method to search for the material foundation of TCMs [12,13,14] via the relationships between TCM fingerprint peaks and specific pharmacodynamic data analyzed by the chemometrics [15], containing hierarchical cluster analysis (HCA), principal component analysis (PCA), the analytic hierarchy process (AHP), stepwise regression analysis (SRA), canonical correlation analysis (CCA), grey relational analysis (GRA), bivariate analysis (BA), multivariate correlation analysis (MCA), etc. (Figure 1). As shown in Table 1, there were two spectrum models (in vitro chemical fingerprint [16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42] and in vivo serum fingerprint [43]) analyzed by capillary electrophoresis (CE), infrared spectroscopy (IR) or liquid chromatography (LC) tandem ultraviolet spectrometry (UV), evaporating light scattering detector (ELSD), flow injection chemiluminescence (FICL) and mass spectrometry (MS) [16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45], and two pharmacodynamic models (in vitro and in vivo) in the spectrum-effect relationship. Among them, the chemical fingerprint was obtained from sample preparations for different batches [16,17,18,19,20,21,22,23,24,25,26,27,28,29], different parts [30,31], different combinations [32,33,34], different ways of processing [35,36,37,38,39,40,41], and different agronomic and environmental parameters [42]. For example, Liu et al., 2014 [19], studied the fingerprints of 10 batches of Radix Astragali by high performance liquid chromatography (HPLC)-diode array detector (DAD)-ELSD and their anti-gastric ulcer effects evaluated by growth-promoting efficacy in GES-1 cells, and found that ononin, astragaloside III, and astragaloside IV in 16 common peaks were the most correlated with effects by GRA, which provided a theoretical foundation for quality control of Radix Astragali. Sun et al., 2013 [30], showed different parts of the fingerprint of Aconitum L. plants (Radix Aconiti Kusnezoffii, Radix Aconiti Lateralis Preparata, and Radix Aconiti Brachypodi, Radix Aconiti, Radix Aconiti Singularis) using ultra-performance liquid chromatography (UPLC)-photodiode array detector (PDA) and demonstrated their anti-bacterial (Escherichia coli) activity by micro-calorimetry, and found that hypaconitine and two unknown components (peaks 1 and 3) might be the most important ingredients by using CCA. Bao et al., 2014 [32], reported the fingerprints of 20 combinations in Qizhiweitong granules composed of Radix bupleuri, Rhizoma corydalis, Fructus Aurantii, Rhizoma cyperi, Radix Paeoniae Alba, and Radix glycyrrhizae preparata using HPLC-DAD and their promoting effect on the gastro-intestine evaluated by cyclic guanosine monophosphate (cGMP) and nitric oxide (NO) levels in small intestinal smooth muscle cells, and found that naringin, neohesperidin, hesperidin, neoponcirin, narirutin, liquiritin apioside, albiflorin analogues, neoeriocitrin, and glycyrrhizin were the active components analyzed by the GRA and back propagation (BP) neural network. Zheng et al., 2014 [39], showed the spectrum-effect relationship between the UPLC fingerprint of crude secondary roots of Aconitumcarmichaelii Debeaux (FuZi) and its three processed products and their mitochondrial growth (micro-calorimetricmeasurement) analyzed by CCA, and found that benzoylhypacoitine, benzoylaconitine, and mesaconitine might be the main active ingredients. Liu et al., 2014 [43], reported the serum fingerprint at different time points after oral administration of Da-Huang-Fu-Zi-Tang (Rheum officinale Baill., Aconitum carmichaelii Debx., and Asarum sieboldii Miq.) by ultra high performance liquid chromatography-electrospray ionization-quadrupole-time of flight-mass spectrometry (UHPLC-ESI-Q-TOF-MS) and their effect on pancreatic acinar cells (AR42J) from injury, and found that rhein isomer methylation, rhein glucoside, hydroxyl-chrysophanol, hypaconine, talatisamine, chysophanol glucuronide conjugation, and chysophanol glucuronide conjugation might be the principle constituents analyzed by CCA.
Figure 1.
The spectrum-effect relationships for Traditional Chinese medicines (TCMs).
Table 1.
Study on the spectrum-effect relationships for Traditional Chinese medicines (TCMs).
| Names | TCMs Composition | Fingerprint | Pharmacology | Experimentalmodel | Analytical Method | Active Components | Reference |
|---|---|---|---|---|---|---|---|
| Cichorium intybus L. | - | HPLC-DAD-MS | Anti uric acid | Quails | CCA | Aesculin, chlorogenic acid, chicoric acid, isochlorogenic acid A/B/C and 13,14-seco-stigma5(6), 14(15)-diene-3α-ol | [16] |
| Tripterygium glycosides | Tripterygium wilfordii | HPLC | Anti-inflammatory, immunosuppressive activities | mice spleen cells | GRA | Peak 5, peak 10 | [17] |
| Radix Astragali | - | HPLC-PDA-ELSD | Anti-gastric effect | Mice, GES-1 cell | GRA | Ononin, astragaloside III, astragaloside IV | [19] |
| Rhizoma Coptidis | - | UPLC-PDA/HPLC-DAD | Antibacterial effect/Anti-MRSA activity/Anti-inflammatory | Escherichia coli/Broth microdilution/RAW264.7 mouse macrophage cells | HCA, CCA, PCA, PLS | Berberine, jateorrhizine, palmatine, coptisine, epiberine | [18,20,28] |
| Da Cheng Qi Tang | Rhizoma Rhei, Cortex Magnoliae officinalis, Fructus Aurantii Immaturus | HPLC-DAD | Purgative effect | Mice | HCA | Hesperidin, aloe-emodin, honokiol, rhein, magnolol, emodin, sennoside A | [21] |
| Polygonum cuspidatum | - | HPLC-DAD-FICL | Anti-oxidant effect | H2O2 scavenging activities | CA | Piceid, resveratrol, torachrysone-8-O-glucoside, questin/physcion, peak1, peak 10 | [22] |
| Acalypha australis Linn. | - | UPLC/MS, semi-preparative HPLC | Antibacterial effect | Agar-diffusion method; Broth microdilution method | - | Gallic acid, peak 6, peak 9–11 | [23] |
| Zathoxylum nitidum | - | IR | Antitumor effect | 7901, Hela cells | MLR | Nitidine chloride | [24] |
| Morinanepalensis | - | HPLC-ELSD | NO inhibition | RAW264.7 cell | PLS | Peak 2, peak 4–6, peak 10, peak 12, peak 13 | [25] |
| Rheum species | - | UPLC-PDA | Anti-HIV activity(Ribonuclease H) | enzyme activity | BA | Catechin, epicatechin, aloe-emodinmonoglucoside, Peak (tR = 21.28 min) | [26] |
| Rabbiteye blueberry | - | HPLC-DAD | Antioxidant effect | DPPH radical scavenging | HCA | Delphinins, anthocyanidin-3-glucosides | [27] |
| EtOAC extracts of Radix Isatidis | - | HPLC-DAD | Antibacterial effect | Escherichia coli | HCA, MLR, PCA | Salicylic acid | [29] |
| Radix Aconiti, Radix Aconiti Singularis, Radix Aconiti Kusnezoffii, Radix Aconiti Lateralis Preparata, Radix Aconiti Brachypodi | - | UPLC-PDA | Antibacterial effect | Escherichia coli | CCA | Hypaconitine, peak 1, peak 3 | [30] |
| Polygonum orientale | - | UPLC-PDA | Anti-oxidative injury | H9c2 myocardial cell | BA | Peak 3–5, peak 11–14, peak 18, peak 19, peak 21–25 | [31] |
| Qizhiweitong Granules | Radix bupleuri, Rhizoma Corydalis, Fructus Aurantii, Rhizoma Cyperi, Radix Paeoniae Alba, Radix glycyrrhizae Preparata | HPLC-DAD | Promoting gastrointestinal motility | Small intestine smooth muscle cells | GRA, BP neural network | Naringin, neohesperidin, hesperidin, neoponcirin, narirutin, liquiritinapioside, albiflorin analogues, neoeriocitrin, glycyrrhizin | [32] |
| ZuoJin Wan | Coptis chinensis Franch.Evodia rutaecarpa (Juss.) Benth. | HPLC-DAD | Biothermo-logical effect | Escherichia coli | CCA | Evodiamine, palmatine hydrochloride, berberine hydrochloride | [33] |
| Suanzaoren decoction | Semen Ziziphi Spinosae, poria, rhizoma Chuanxiong, rhizome Anemarrhenae, radix glycyrrhizae | HPLC-PDA | Sedative effect | Mice | Correlation and regressive analysis | Spinosin, ferulic acid, mangiferin, glycyrrhizic acid, peak 3, peak 8, peak 9, peak 16, peak 21, peak 34, peak 42, peak 46, peak 47 | [34] |
| Platycladi cacumen | - | HPLC-MS/MS | Hemostatic activities | New Zealand rabbit | CCA | Cecarbon | [35] |
| Radix Hedysari | - | HPLC | Anti-hepatic fibrosis | Mice | GRA, PLS, | Adenosine, calycosin | [36] |
| Saffron | - | HPLC-DAD | Antioxidants | DPPH | MCA | Crocins-1, crocins-2, crocins-3 | [37] |
| Flos Sophorae | - | HPLC-MS/MS | Hemostatic activities | New Zealand rabbit | CCA | Huaicarbon A, huaicarbon B | [38] |
| Aconitum carmichaelii Debeaus | - | UPLC-ELSD | Mitochondria growth promoting effect | Rat | CCA | Mesaconitine, benzoylaconitine, benzoylhypacotine | [39] |
| Artificial Calculus bovis | - | UPLC-ELSD | Antibacterial effect | Escherichia coli | HCA, MLR, PCA | Cholic acid, taurocholate sodium, chenodeoxycholic acid | [40] |
| Belamcanda chinensis leaf | - | HPLC-DAD | Hypoglycemic effect | Rat | - | Flavonoids (tectoridin, swertisin) | [41] |
| Da-Huang-Fu-Zi-Tang | Rheum officinale Baill., Aconitum carmichaelii Debx., Asarum sieboldii Miq. | UHPLC-ESI-Q-TOF-MS | Anti-acute pancreatitis effect | AR42J cell | CCA | Talatisamine, rhein glucoside, rhein isomer methylation, hypaconine, hydroxyl-chrysophanol, emodin glucuronide conjugation, chysophanol glucuronide conjugation | [43] |
CCA: canonical correlation analysis; GRA: grey relational analysis; HCA: hierarchical cluster analysis; PCA: principal component analysis; PLS: partial least squares; MLR: Multiple linear regression ; BA: bivariate analysis ; BP: back propagation; MCA: multivariate correlation analysis; CE: capillary electrophoresis; IR: infrared spectroscopy; LC: liquid chromatography; UV: ultraviolet spectrometry; ELSD: evaporating light scattering detector; FICL: flow injection chemiluminescence; MS: mass spectrometry.
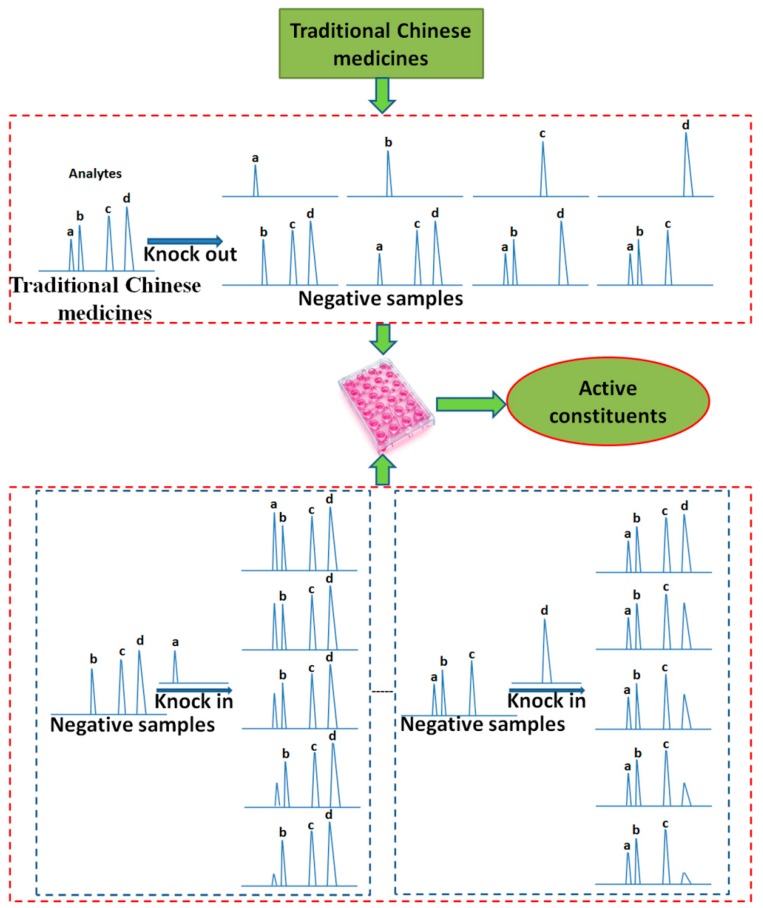
2.3. Knock-in and Knock-out
Xiao et al., 2009 [45], put first forward that constituents knock-out/knock-in, inspired by functional genetic methods, are novel patterns of efficient component recognition and quality control for TCMs, which include marker compounds identified by studying the effect of the constituents knocked out on efficacies, and the dosage-effect or dosage-toxicity relationships studied by observing the effect of marker compounds knocked in on efficacies (Figure 2; Table 2). For example, Yan et al., 2014 [46], and Li, 2013 [47], reported the identification of the major active constituents for bacterial diarrhea treatment evaluated by the growth of Shigella dysenteriae using microcalorimetry in Rhizoma coptidis by the knock-out and knock-in method, and found that coptisine and berberine were the important components with bacteriostatic activities of 54.10% and 39.75%, respectively, by the knock-out method, and their suitable concentration ranged from 8.08% to 31.92% and from 4.05% to 14.45% of the total, respectively, by the knock-in method. Jin et al., 2013 [48], showed the identification of bioactive compounds for osteoporosis treatment evaluated by osteoblasts cell proliferation and differentiation in Herba Epimedii by the knock-out method, and found that epimedin A, epimedin B, epimedin C, and icariin were the main active constituents. Yu et al., 2009 [49], studied the assessment of effective components for anti-tumor activity evaluated by the synergistic effects of cyclophosphamide on chemotherapy for S180 tumor-bearing mice in Shenmai formulae composited of Radix Ginseng and Radix Ophiopogonis by the knock-out method, and found that panoxadiol and a type of ginseoside were the active components.
Figure 2.
The knock-in/knock-out model for TCMs (a, b, c, and d represent different components in TCMs).
Table 2.
Study on the knock-in and knock-out target components in TCMs.
| Names | Knock-in or Knock-out Components | Pharmacology | Experimental Model | Active Components | Reference |
|---|---|---|---|---|---|
| Rhizoma Coptidis | Berberine, palmatine, coptisine, epiberberine, jateorrhizine, columbamine | Growth inhibition of shigelladysenteriae | Microcalorimetry | Berberine, coptisine | [46,47] |
| Herba Epimedii | Epimedin A, epimedin B, epimedin C, icariin | Cell proliferation, differentiation | Third generation rat osteoblasts | Epimedin A, epimedin B, epimedin C, icariin | [48] |
| Calculus bovis | Bilirubin, bilirubin conjugate, glycocholic acid, cholic acid, chenodeoxycholic acid, hyodeoxycholic acid, sodium taurocholic acid, deoxycholic acid | Inhibition of hydrogen peroxide-induced damage | SH-SY5Y | Bilirubin, bilirubin conjugate, glycocholic acid, cholic acid | [50,51] |
| Flos Lonicerae Japonicae | Isochlorogenic acids, chlorogenic acid, flavones, iridoid glycosides | Anti-virus, anti-bacteria | Vero cell, Escherichia coli | Isochlorogenic acids | [52] |
| Rhizoma Curcumae Longae | Curcumin, demethoxycurcumin, bisdemethoxycurcumin | Anti-oxidant activity, anti-coagulant effect, anti-oxidant stress damage | DPPH, rabbit, PC12 | Curcumin > demethoxycurcumin > bisdemethoxycurcumin | [53,54] |
| Radix puerariae | Puerarin, daidzin, daidzein, compound X | Anti-oxidant damage | HUVEC | Puerarin, compound X | [55] |
| Shenmai formulae | Panoxadiol, panaxotriol, ophiopogonpolysaccharide, ophiopogonin | Antitumor effect | S180 bearing mice | Panoxadiol, panaxotriol, ophiopogonpolysaccharide | [49] |
HUVEC: human umbilical vein endothelial cells; DPPH: 2,2-diphenylpicrylhydrazyl; PC12: pheochromocytoma.
We found above that the knock-in method can be suitable for identifying the effective components in Chinese herbal extracts and Chinese herbal compounds, but the application for the knock-out method is limited due to the fact that the target constituents are difficult to remove from Chinese herbal compounds.
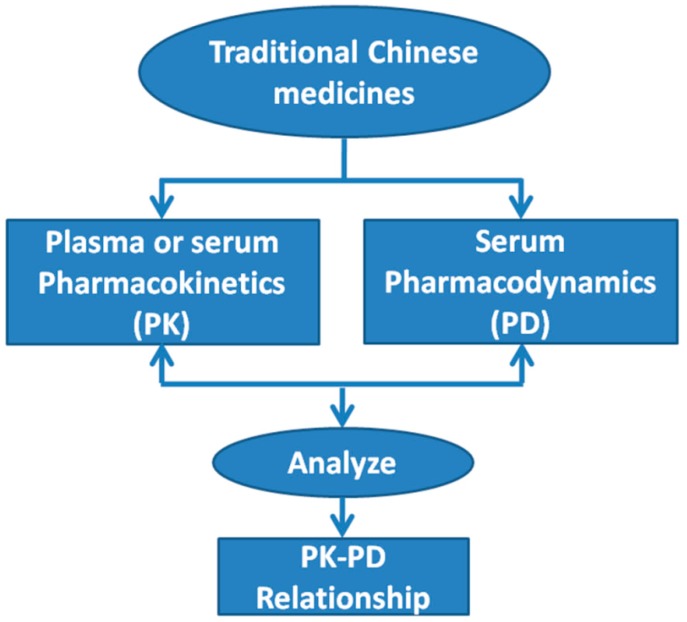
2.4. Pharmacokinetics (PK)-Pharmacodynamics (PD)
Pharmacokinetics (PK)-pharmacodynamics (PD), put forward first by Sheiner et al., 2009 [56], are extensively applied for effective constituent identification in the field of TCMs, which mainly includes the correlation analysis between PK (the blood-drug concentration method) and PD (the pharmacology-effect method) (Figure 3 and Table 3). For example, Liu et al., 2014 [43], reported the PK profiles of multiple components after oral administration of Da-Huang-Fu-Zi-Tang and the PD profiles evaluated by the effect of the serum at different time points on pancreatic acinar cells (AR42J) from injury, and found that rhein isomer methylation, rhein glucoside, hydroxyl-chrysophanol, hypaconine, talatisamine, chysophanol glucuronide conjugation, and chysophanol glucuronide conjugation might be the principle constituents analyzed by CCA. Peng, 2014 [57], studied the PK of baicalin, geniposide, cholalic acid, hyodeoxycholic acid, chlorogenic acid, and neochlorogenic acid in a Qingkailing injection composed of Cholalicacid, Conchamargaritifera, Hyodeoxycholic acid, Gardeniae Fructus, Cornububali, Radix isatidis, Baicalin, and Flos Lonicerae Japonicae using UPLC-ESI-MS/MS and studied the PD by evaluating temperature changes in rats, and found that baicalin and geniposide were the main effective ingredients by using Winnonlin software analysis. Wang et al., 2014 [58], showed that Tanshinone IIA was the main ingredient for anti-oxidant activity in a Yin-Teng-Gu-Bi-Kang prescription composed of Radix Salviae Miltiorrhiae, Angelicae Sinensis Radix, Paeoniae Radix Alb, and Celastrusorbiculatus Thunb. analyzed by the PK (Tanshinone IIA concentration in plasma)-PD (malondialdehyde (MDA) level in serum) model.
Figure 3.
The pharmacokinetics (PK)-pharmacodynamics (PD) relationships for TCMs.
Table 3.
Study on the pharmacokinetics (PK)-pharmacodynamics (PD) relationships in TCMs.
| Names | TCMs Composition | PK Ingredients | PD | Analytical Method | Active Components | Reference |
|---|---|---|---|---|---|---|
| Da-Huang-Fu-Zi-Tang | Rheum officinale Baill., Aconitum carmichaelii Debx., Asarum sieboldii Miq. | Talatisamine emodin isomer | Anti-acute pancreatitis effect in AR42J cell | CCA AUE-lgAUC E-logC Winnonlin | Talatisamin chysophanol glucuronide conjugation | [43] |
| Qingkailing injection | Cholalic acid, Concha margaritifera, Hyodeoxycholic acid, Gardeniae Fructus, Cornu bubali, Radix isatidis, Baicalin, Flos Lonicerae Japonicae | Baicalin, geniposide, cholalic acid, hyodeoxycholic acid, chlorogenic acid, neochlorogenic acid | Temperature changes in rat | Baicalin, geniposide | [57] | |
| Yin-Teng-Gu-Bi-Kang Precription | Radix Salviae Miltiorrhiae, Angelicae Sinensis Radix, Paeoniae Radix Alb, Celastrus orbiculatus Thunb. | Tanshinone IIA | MDA in rat’s serum | Tanshinone IIA | [58] | |
| Shengmai injection | Red ginseng, ophiopogon japonicas (Thunb.) Ker-Gawl, schisandra chinensis | Ginsenoside (Rg1, Rb1) | NO in rat’s serum | Ginsenoside (Rg1, Rb1) | [59] | |
| Rhizoma Curculiginis | — | Orcinol glucoside | SOD, GSH, GSH-PX in plasma | Orcinol glucoside | [60] | |
| Schisandra chinensis alcoholic extract | — | Schisandrin, gomisin D, gomisin O, tigloylgomisin H, angeloylgomisin Q, gomisin G, gomisin B, angeloylgomisin P, schisantherin A, gomisin E, schisantherin D, deoxyschizandrin, gomisin R, γ-schisandrin, angeloylisogomisin O, angeloylgomisin O, 6-O-benzoyl gomisin O, 7-8-dihydroxy-schizandrin, PeaktR (42.0 min) | ALT in rat’s serum | Schisandrin, schisantherin A, deoxyschizandrin, γ-schisandrin, 7-8-dihydroxy-schizandrin, PeaktR (42.0 min) | [61] | |
| Radix et Rhizoma Rhei | — | Aloe Emodin, rhein, emodin, chrysophanol | Amylase, endotoxin, TNF-α, diamineoxidase in beagle dog’s serum; Temperature changes and NO in rat in vivo | Rhein | [62] | |
| Tea polyphenols | — | Epigallocatechingallate, epicatechingallate, epigallocatechin, epicatechin | MDA in rat’s liver | Epigallocatechi-n gallate, epicatechingallate, epigallocatechi-n, epicatechin | [63] |
SOD: superoxide dismutase; GSH: glutathione; GSH-PX: glutathione peroxidase; ALT: alanine transaminase; MDA: malondialdehyde; AUE: area under efficacy; AUC: area under concentration. E: efficacy; C: concentration.
3. Evaluation System of Bioavailability Establishment for TCMs
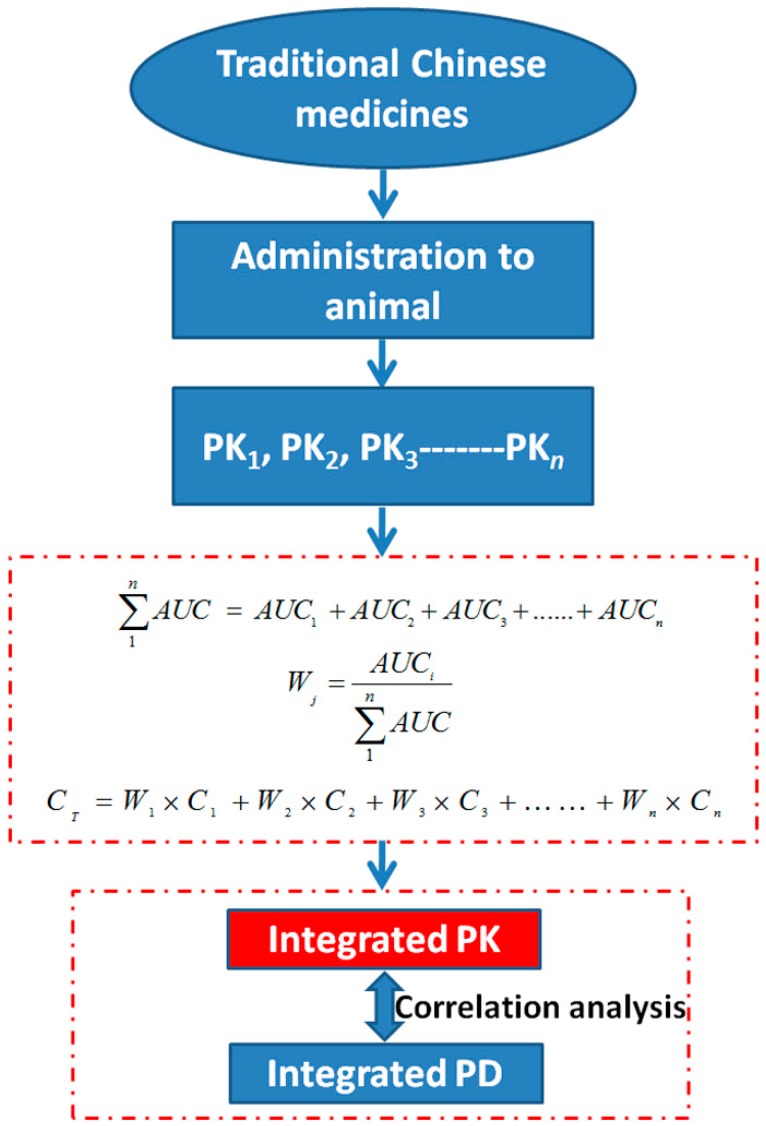
The construction of the evaluation system of bioavailability is one of the most important scientific issues in the modernization of TCMs. Hao et al., 2009 [64], first reported that an area under curve (AUC)-weighting method could obtain the integral PK properties based on the same type of components in TCMs (Figure 4). The weighting coefficient for each constituent was calculated using Equations (1) and (2). The integral concentrations (CT) at each time point were then calculated by Equation (3), where w represented the weighting coefficient, AUC1–AUCn represented bioavailability in vivo and C1–Cn represented the plasma concentration of each constituent studied. The evaluation system establishment could comprehensively estimate the correlation between integral PK and PD, especially for the TCMs with a narrow therapeutic window, to ensure safety in practical applications. As seen in Table 4, Dong et al., 2014 [65], showed the integral PK profiles of Rhodojaponin I, II, and III, the active components in Rhododendri Mollis Flos, and found that the correlation with the potential markers of myocardial injury ((creatine kinase-measurement blood) (CK-MB) and lactate dehydrogenase (LDH)) was fairly strong, which can be conductive to fully understanding the relationship between the PK behaviors and the compound’s efficacy. Guo et al., 2014 [66], and Li et al., 2008 [67], successfully developed the integral PK profiles in the plasma and brain of ginsenosides Rg1, Rb1, Re, Rd, and panax notoginsenoside R1, the main active components in Panax notoginseng (Burk.) F.H.Chen (Sanqi). Pan et al., 2014 [68], and Zhu et al., 2012 [69], showed the integrated PK of baicalin, baicalein, geniposide, palmatine, and berberine, the main effective ingredients in Huang-Lian-Jie-Du-Tang in middle cerebral artery occlusion (MCAO) rats, and found that the correlation with the anti-ischemia index (Interleukin 6 (IL-6), tumor necrosis factor (TNF-α), superoxide dismutase (SOD), glutamic acid (Glu), and MDA) in the serum was good, which would provide comprehension better understanding of cerebrovascular disease as Huang-Lian-Jie-Du-Tang is used in clinical practice. Xie et al., 2010 [70], reported the holistic PK of Schisandrin, schisantherin A, deoxyschisandrin, and γ-schisandrin, the four main lignin components in Schisandra, and found that the integral AUC and CYP3A activities correlated well with hepatic injury biomarkers (ALT and aspartate aminotransferase (AST)) in serum. However, the integral PK calculated by an AUC-weighting method was established on the basis of the fact that the bioavailability of the integral components was positively correlated with their efficacy. For example, the AUC value of compound A was higher than that of compound B, but their efficacy was opposite. It means that the effect of the bioavailability fluctuation of compound B on the integral AUC was far less than that of compound A, but that its effect on the pharmacology was far stronger than that of compound A, which resulted in the integral PK parameters being negatively or not correlated with pharmacology. Therefore, an AUC-weighting method might not be well suited for studying the integral PK of all TCMs. It was presumed that efficacy as a weight coefficient might be more reasonable if the efficacy we chose could represent the pharmacological effects of TCMs.
| (1) |
| (2) |
| (3) |
Figure 4.
The integrated pharmacokinetics for TCMs.
Table 4.
Study on the integrated pharmacokinetics in TCMs.
| Names | TCMs Composition | Integrated Ingredients | Integrated Method | Pharmacology | Correlation Analysis | Reference |
|---|---|---|---|---|---|---|
| Rhododendri Mollis Flos | - | Rhodojaponin (I, II, III) | Weighting factor based on AUC | Myocardial damage (LDH, CK-MB) | - | [65] |
| Panax Notoginseng Saponins1 | - | Panax Notoginsenoside R1, Ginsenosides Rg1, Rb1, Re, Rd | - | - | [66,67] | |
| Huanglian-Zhizi couplet medicine | Rhizoma Coptidis, Fructus Gardeniae | Gardenia acid, geniposide | Antioxidant efficacy (SOD) | E-C | [68] | |
| Huang-Lian-Jie-Du-Tang | Rhizomacoptidis, Radix scutellariae, Cortex phellodendri, Fructusgardeniae | Berberine, palmatine, baicalin, baicalein, geniposide | Anti-ischemia | - | [69] | |
| Schisandra lignans | - | Schisandrin, schisantherin A, deoxyschisandrin, γ-schisandrin | Serum alanine aminotransferase (ALT), aspartate aminotransferase (AST) | E-C | [70] | |
| Jiao-Tai-Wan | Rhizomacoptidis powder, Cortex cinnamomi powder | Berberine, palmatine, coptisine, epiberberine, jatrorrhizine | - | - | [71] | |
| Huang-Lian-Jie-Du-Tang | Rhizomacoptidis, Radix scutellariae, Cortex phellodendri, Fructusgardeniae | Groenlandicine, berberine, palmatine, epiberberine, jatrorrhizine, columbamine | - | - | [72] | |
| Total coumarins in Radix Angelicae dahuricae | - | Bergapten, imperatorin…isoimperatorin | - | - | [73] | |
| Tea polyphenols | - | Epigallcocatechingallate, Epicatechingallate, Epigallocatechin, Epicatechin | Anti-lipid peroxidation in vitro of mouse liver homogenate | E-logC | [74] | |
| Gegen-Qinlian Decoction | Radix Puerariae, Radixscutellariae, Coptidisrhizome, Radixglycyrrhizae | Puerarin, Daidzein, Baicalin, Baicalein, Wogonoside, Wogonin, Glycyrrhizin, Liquiritin, Berberine, Jateorhizine, Palmatine | - | - | [75] |
LDH: lactate dehydrogenase; CK-MB: creatine kinase-measurement blood; E-C: effect-concentration.
4. Pharmaceutical Technology Applications to Improve the Bioavailability of Active Components in TCMs
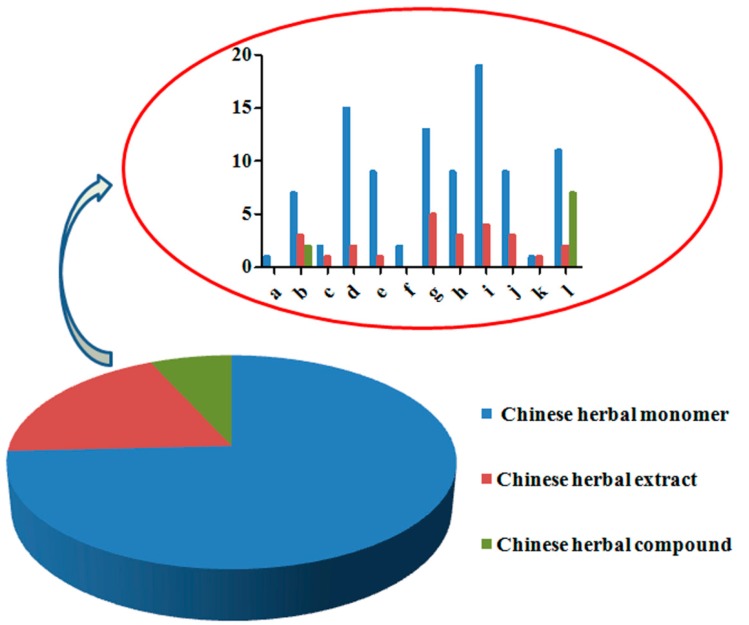
As we all know, the low oral bioavailability of TCMs will contribute to their poor clinical therapeutic effects. However, formulation designation and pharmaceutical technology applications are severely disrupted by the complex physico-chemical properties for both active ingredients and their associated constituents in TCMs. As reported in the PubMed Database (2006–current), the pharmaceutical methods applied to TCMs classified II (high permeability and low solubility) in the Biopharmaceutics Classification System (BCS) [76] included mainly micronization [77,78], nano-suspensions [79,80,81,82,83,84,85,86,87,88,89,90], solid dispersion [91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108], phospholipid complex [92,109,110,111,112,113,114,115,116,117,118,119,120], β-cyclodextrin complex [121,122,123,124,125,126,127,128,129,130,131], microemulsion [132,133,134,135,136,137,138,139,140,141,142,143,144,145], self-microemulsion [146,147,148], and polymeric micelles [149,150], etc. The pharmaceutical methods applied to TCMs classified III (low permeability and high solubility) [76] included mainly microemulsion [132,133,134,135,136,137,138,139,140,141,142,143,144,145], liposome [151,152,153,154,155,156,157,158,159,160,161,162,163,164,165,166,167,168,169,170,171,172], lipid nanoparticles [173,174,175,176,177,178,179,180,181,182,183,184,185,186,187,188,189,190], bioadhesive polymer [191], absorption enhancers [192,193,194,195,196,197,198,199,200,201,202,203,204,205,206], etc. According to the statistics, the proportions of the pharmaceutical technologies applied to Chinese herbal monomers, Chinese herbal extracts, and Chinese herbal compounds, respectively, were 74.24%, 18.94%, and 6.82% (Figure 5); the percentage of Chinese herbal compounds using absorption enhancers was 77.78% compared with those using other methods (Figure 5), which indicated that the absorption enhancers should be considered the preferred pharmaceutical technology in Chinese herbal compound preparations, such as the preparations recorded in Chinese Pharmacopeia (Volume I) [207] as the active constituents recognized as belonging to those classified III in the BCS [76].
Figure 5.
The applications of related pharmaceutical technologies for Chinese herbal monomers, extracts and compound. (a: bioadhesive polymers; b: mircoemulsion; c: self-microemulsion; d: nanosuspensions; e: polymer β-Cyclodextrin inclusion; f: polymeric micelles; g: liposomes; h: phospholipid complex; i: solid lipid nanoparticles; j: solid dispersion; k: micronization; l: absorption enhancer).
5. Study on the Evaluation System of Bioavailability Establishment and Related Pharmaceutical Technologies—Flos Lonicerae Japonicae—Fructus Forsythiae Herb Couples as an Example
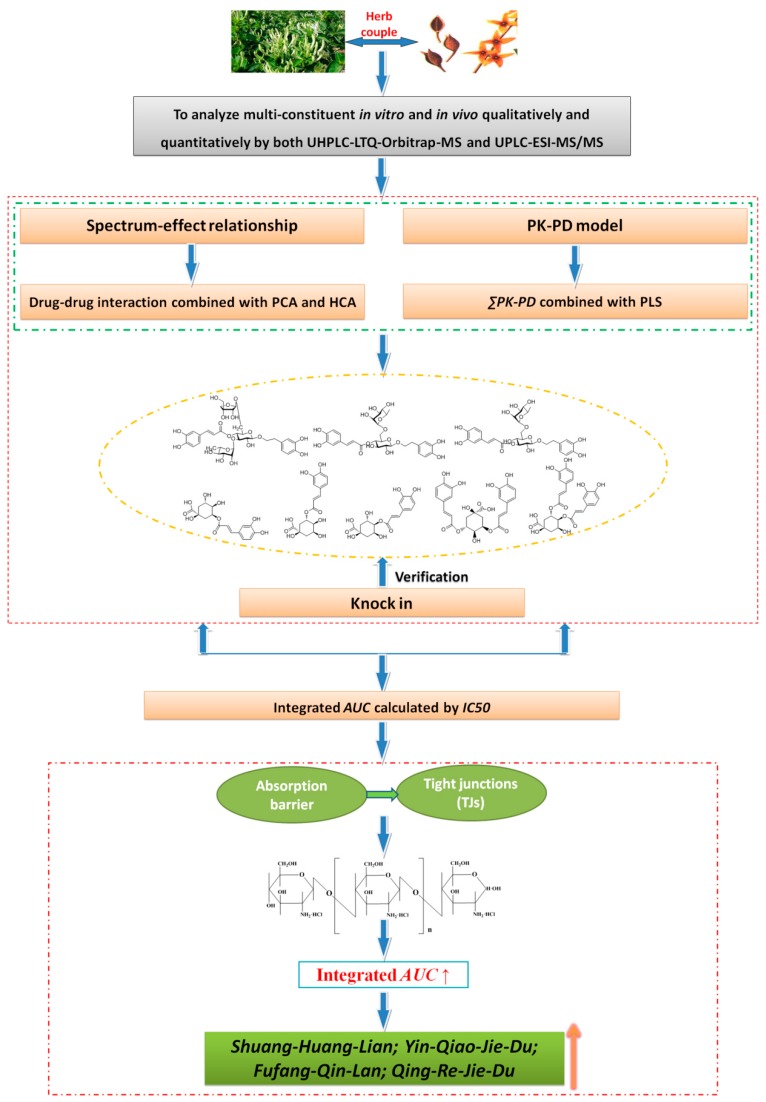
We have previously studied the evaluation system of bioavailability establishment and related pharmaceutical technology applications based on the Flos Lonicerae Japonicae-Fructus Forsythiae (FLJ-FF) herb couple as a model drug (Figure 6).
Figure 6.
The evaluation system of bioavailability establishment and related pharmaceutical technology applications for the Flos Lonicerae Japonicae-Fructus Forsythiae (FLJ-FF) herb couple (↑: improvement).
Firstly, the qualitative and quantitative methods in vitro and in vivo for the multi-constituents in the FLJ-FF herb couple were established. We found 35 components in vitro using UHPLC-LTQ-Orbitrap-MS, including seven phenolic acids, five phenylethanoid glycosides, seven flavones, two isoflavones, nine lignans, two saponins, and three iridoids, and 26 ingredients (neochlorogenic acid, chlorogenic acid, cryptochlorogenic acid, 3,5-dicaffeoylquinic acid, 3,4-dicaffeoylquinic acid, caffeic acid, quinic acid, isoforsythoside, forsythoside A, forsythoside B, rutin, luteolin, astragalin, hyperoside, isoquercitrin, quercetin, luteoloside, genistin, genistein, arctiin, phillyrin, pinoresinol-β-d-glucoside, arctigenin, dipsacoside B, macranthoidin B, and loganin) were quantified simultaneously by UPLC-ESI-MS/MS [208]. Meanwhile,32 components in vivo (29 prototype compounds and three metabolites) were identified by UHPLC-LTQ-Orbitrap-MS with MetWorks software, which included seven phenolic acids, five phenylethanoid glycosides, seven flavones, two isoflavones, seven lignans, one iridoid, and three metabolites (pinoresinol-O-glucuronide, epipinoresinol-O-glucuronide, and phillygenin-O-glucuronide), and 23 ingredients (neochlorogenic acid, chlorogenic acid, cryptochlorogenic acid, 3,5-dicaffeoylquinic acid, 3,4-dicaffeoylquinic acid, caffeic acid, quinic acid, isoforsythoside, forsythoside A, forsythoside B,rutin, luteolin, astragalin, hyperoside, isoquercitrin, quercetin, luteoloside, genistin, genistein, phillyrin, pinoresinol-β-d-glucoside, and arctigenin) were quantified simultaneously by UPLC-ESI-MS/MS [209].
Secondly, both drug-drug interaction (DDI) (spectrum-effect relationship) [208] and ΣPK-PD (PK-PD model) [210] were simultaneously performed to identify the chemical markers in the FLJ-FF herb couple, and they were verified by the “knock-in” method (data not shown). The result showed that caffeic acid derivatives(neochlorogenic acid, chlorogenic acid, cryptochlorogenic acid, 3,5-dicaffeoylquinic acid, 3,4-dicaffeoylquinic acid, isoforsythoside, forsythoside A, forsythoside B) can be considered as marker compounds in the FLJ-FF herb couple.
Thirdly, the integral PK for caffeic acid derivatives based on an AUC-weighting approach was established, but the bioavailability of chlorogenic acids was negatively correlated with their efficacy. For example, the AUC of chlorogenic acid in the FLJ-FF herb couple was much higher than that of forsythoside A, but the IC50 was lower than that of forsythoside A (data not shown). It meant that the effect of the bioavailability fluctuation of phenylethanoid glycosides on the integral AUC calculated by an AUC-weighting approach was far less than that of the chlorogenic acids, but the effect on the pharmacology was far more than that of the chlorogenic acids, which resulted in the antiviral activity, not the integral AUC, being improved significantly as forsythoside A knocked in the FLJ-FF herb couple (data not shown). However, the integral AUC calculated by IC50 as follows (W represents the weighting coefficient and the C1–Cn represents the plasma concentration of the components studied) was increased gradually as the antiviral activity was improved by the FLJ-FF herb couple knocked-in forsythoside A, showing a strong positive correlation, and the integral PK parameters using IC50 as the weight coefficient index could fully take eight caffeic acid derivatives’ PK parameters into account (data not shown). The results above indicated that IC50 as a weight coefficient was more reasonable than AUC.
| (4) |
| (5) |
| (6) |
Finally, the antiviral activity of commercially available FLJ-FF herb couple preparations (Shuang-Huang-Lian oral liquid, Yin-Qiao-Jie-Du tablet, Fufang-Qin-Lan oral liquid, and Qing-Re-Jie-Du oral liquid) was regulated based on the integrated AUC calculated by IC50. The antiviral effect was decreased significantly as the four preparations knocked out the FLJ-FF herb couple, but increased significantly as the FLJ-FF herb couple was knocked in (data not shown). Besides, the integral absorption of caffeic acid derivatives in the four preparations was improved significantly both in vitro and in vivo by the chito-oligosaccharide (COS) (data not shown), which was consistent with the fact that the absorption of caffeic acid derivatives in monomers, the FLJ-FF herb couple, or its preparations was mainly restricted by tight junctions (TJs) [211,212,213,214], and COS was an absorption enhancer based on tight junctions with high effectiveness and low mucosal toxicity [215]. In addition, the treatment with FLJ-FF herb couple preparations with COS can restrain the MDCK cell damage upon influenza virus propagation better than that of the control [216], but the treatment with the preparations with the COS-knocked-out FLJ-FF herb couple showed non-significance compared to that of control (data not shown). The results above illustrated not only the antiviral activity improvement due to the COS in FLJ-FF herb couple preparations resulting from the improvement of the integrated AUC of caffeic acid derivatives, but also showed the reasonability of the weight coefficient calculated by IC50, not AUC. Absorption-enhancer COS has been successfully applied for the second development of FLJ-FF herb couple preparations.
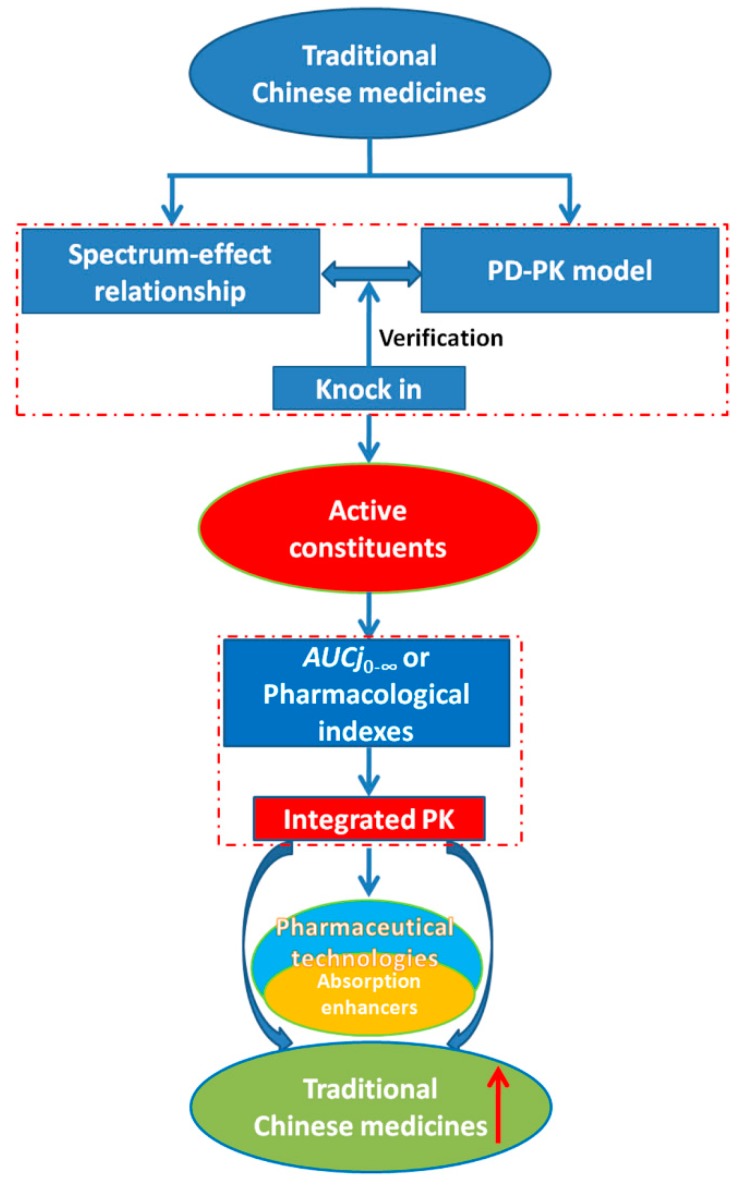
6. Conclusions and Future Perspective
TCM preparations, extensively recorded in Chinese Pharmacopoeia, have long history with applications for protecting health and controlling disease [207]. The present quality assessment of TCM preparations mainly focused on single chemical constituents, not biological indicators, as markers, and novel pharmaceutical excipients were hardly applied for TCM preparations due to their complicated physico-chemical properties, which resulted in poor effects in clinical practice. Here, we attempted to propose a plan (Figure 7) to deal with the obstacles in order to carry out the bio-pharmaceutical explorations of TCM preparations better. Firstly, both the spectrum-effect relationship and PK-PD model can be simultaneously performed to identify the chemical markers and to be verified by the “knock-in” method; Secondly, the weight coefficient calculated by AUC or the efficacy should be compared to decide which one is more suitable for the integral PK; Thirdly, an absorption enhancer might be considered the preferred pharmaceutical technology in Chinese herbal compound preparations, such as the preparations recorded in Chinese Pharmacopeia (Volume I) as the active constituents recognized as belonging to those classified III in the BCS [207].
Figure 7.
The path for studying bio-pharmaceutics for TCM preparations (↑: improvement).
In recent years, LC-MS was rapidly accepted by the analytical community, and it was gradually applied for qualitative and quantitative analysis [217,218,219,220], PK study [221,222], metabolite in vivo identification [223,224], metabolomics [225,226], quality control [227,228], and pharmacological studies [229,230] in TCMs. The novel methods, such as aggregation morphology [231] and magnetic molecularly imprinted polymer [232], were also helpful in understanding the mechanism of TCMs and discovering drugs based on TCMs. Besides, systems biology (genomics, proteomics, metabolomics, and bioinformatics), a new subject in the field of life sciences, provided a comprehensive resource for the modernization and advancement of TCMs as well as general drug discovery efforts, which proposed a system-to-system research methodology to study the interaction between TCMs and the human body and their applications in drug research and development [233]. In addition, some promising excipients can also accelerate the development of TCM preparations. For example, Kollidon CL, manufactured by BASF, the largest chemical producer in the world, can produce the highest disintegration speed (18 min), which is 50% faster than croscarmellose sodium (CMC-Na: 27 min) and almost three times faster than sodium starch glycolate (CMS-Na: 50 min), and which can be applied for surmounting the obstacles of poor solubility and long disintegration times in oral solid dosage forms of TCM preparations such as tablets or capsules when the active constituents recognized belonged to those classified II in the BCS. Chitosan derivatives, such as N-trimethyl chitosan chloride [234] and chito-oligosaccharide [215], synthesized with remarkable solubility at neutral pH in an aqueous environment, were not only non-toxic, biocompatible, and biodegradable, but also performed as intestinal absorption enhancers by reversible opening of the tight junctions, which can be applied for improving the permeability of active constituents as the active constituents recognized belonged to those classified III in the BCS. We expect that the current study will be positive and informative.
Acknowledgments
The present study is supported financially by the National Natural Science Foundation of China (81273655), the “Qing Lan” Project from Jiangsu Provincial Technology Innovation Team Support Scheme, the priority Academic Program Development of Jiangsu Higher Education Institution (ysxk-2010) and the Fourth Phase of the “333” Project from Jiangsu Province (BRA2013201).
Author Contributions
Wei Zhou wrote the paper; conceived and designed the paper: Wei Zhou, Baochang Cai, JinjunShan, Shouchuan Wang and Liuqing Di.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Yi Y.D., Chang I.M. An overview of traditional Chinese medicine herbal formulae and a proposal of a new code system for expressing the formula titles. Evid. Based Complement. Alternat. Med. 2004;1:125–132. doi: 10.1093/ecam/neh019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang D.F. 3.2 billon dollars exports for traditional Chinese medicine in China. J. Trad. Chin. Med. Manag. 2015;23:125. [Google Scholar]
- 3.Zhou Y.L. The clinical application and adverse reaction of Shuang-Huang-Lian preparations, Nei Mongol Journal of Traditional Chinese Medicine. Nei Mongol J. Trad. Chin. Med. 2010;13:101–102. [Google Scholar]
- 4.White N.J. Assessment of the pharmacodynamic properties of antimalarial drugs in vivo. Antimicrob. Agents Chemother. 1997;41:1413–1422. doi: 10.1128/aac.41.7.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hollman A. Drugs for atrial fibrillation. Digoxin comes from Digitalis lanata. BMJ. 1996;312:912. doi: 10.1136/bmj.312.7035.912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matyášová E., Novák J., Stránská I., Hejtmánková A., Skalický M., Hejtmánková K., Hejnák V. Production of morphine and variability of significant characters of Papaver somniferum L. Plant Soil Environ. 2011;57:423–428. [Google Scholar]
- 7.Trung Bui-Khac T., Dupuis N. Process for Extraction and Purification of Paclitaxel from Natural Sources. 6452024 B1. U.S. Patent. 2000 May 26;
- 8.Johnson I.S., Armstrong J.G., Gorman M., Burnett J.P., Jr. The vinca alkaloids: A new class of oncolytic agents. Cancer Res. 1963;23:1390–1427. [PubMed] [Google Scholar]
- 9.Hu L.S. Application of ginkgo leaf total lactones in preparation of medicament for preventing or treating deafness and tinnitus. 102078343 B. C.N. Patent. 2012 Oct 3;
- 10.Mukhtar H., Ahmad N. Tea polyphenols: Prevention of cancer and optimizing health. Am. J. Clin. Nutr. 2000;71:1698S–1702S. doi: 10.1093/ajcn/71.6.1698S. [DOI] [PubMed] [Google Scholar]
- 11.Li R., Yan Z.Y., Li W.J., Xu T., Tan R.A., Pan L., Li Y.M., Ma Y.L. The establishment of chromatographic pharmacodynamics. Educ. Chin. Med. 2002;21:62. [Google Scholar]
- 12.Liang Y., Xie P., Chau F. Chromatographic fingerprinting and related chemometric techniques for quality control of traditional Chinese medicines. J. Sep. Sci. 2010;33:410–421. doi: 10.1002/jssc.200900653. [DOI] [PubMed] [Google Scholar]
- 13.Calixto J.B. Efficacy, safety, quality control, marking and regulatory guidelines for herbal medicine (phytotherapeutic agents) Braz. J. Med. Biol. Res. 2000;33:179–189. doi: 10.1590/S0100-879X2000000200004. [DOI] [PubMed] [Google Scholar]
- 14.Donno D., Beccaro G.L., Cerutti A.K., Mellano M.G., Bounous G. Bud extracts as new phytochemical source for herbal preparations-quality control and standardization by analytical fingerprint. In: Rao V., Rao L.G., editors. Phytochemicals—Isolation, Characterisation and Role in Human Health. InTech; Rijeka, Croazia: 2015. pp. 187–218. [Google Scholar]
- 15.Gad H.A., El-Ahmady S.H., Abou-Shoer M.I., Al-Azizi M.M. Application of chemometrics in authentication of herbal medicines: A review. Phytochem. Anal. 2013;24:1–24. doi: 10.1002/pca.2378. [DOI] [PubMed] [Google Scholar]
- 16.Zhu C.S., Zhang B., Lin Z.J., Wang X.J., Zhou Y., Sun X.X., Xiao M.L. Relationship between high-performance liquid chromatography fingerprints and uric acid-lowering activities of Cichorium intybus L. Molecules. 2015;20:9455–9467. doi: 10.3390/molecules20059455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chi J., Lin B., Liu Z.H., Yang L.N., Liu X.M., Song H.T. Fingerprint and spectrum-effect relationships on Tripterygium glycosides preparation. Zhongguo Zhong Yao Za Zhi. 2015;40:1479–1483. [PubMed] [Google Scholar]
- 18.Li J.Y., Wang X.B., Luo J.G., Kong L.Y. Seasonal variation of alkaloid contents and anti-inflammatory activity of Rhizoma coptidis based on fingerprints combined with chemometrics methods. J. Chromatogr. Sci. 2015;53:1131–1139. doi: 10.1093/chromsci/bmu175. [DOI] [PubMed] [Google Scholar]
- 19.Liu X.H., Zhao L.G., Liang J., Guo L., Yang Y.L., Hu F., Zhu R.J., Feng S.L. Component analysis and structure identification of active substances for anti-gastric ulcer effects in Radix Astragali by liquid chromatography and tandem mass spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2014;960:43–51. doi: 10.1016/j.jchromb.2014.04.020. [DOI] [PubMed] [Google Scholar]
- 20.Luo J.Y., Yan D., Yang M.H. Study of the anti-MRSA activity of Rhizoma coptidis by chemical fingerprinting and broth microdilution methods. Chin. J. Nat. Med. 2014;12:393–400. doi: 10.1016/S1875-5364(14)60049-2. [DOI] [PubMed] [Google Scholar]
- 21.Xie R.F., Zhou X., Shi Z.N., Li Y.M., Li Z.C. Study on spectrum-effect relationship of rhizoma Rhei, cortex Magnoliae Officinalis, fructus Aurantii Immaturus and their formula. J. Chromatogr. Sci. 2013;51:524–532. doi: 10.1093/chromsci/bms172. [DOI] [PubMed] [Google Scholar]
- 22.Ding X.P., Zhang C.L., Qi J., Sun L.Q., Qin M.J., Yu B.Y. The Spectrum-Effect integrated fingerprint of Polygonum cuspidatum based on HPLC-diode array detection-flow injection-chemiluminescence. Chin. J. Nat. Med. 2013;11:546–552. doi: 10.3724/SP.J.1009.2013.00546. [DOI] [PubMed] [Google Scholar]
- 23.Xiao S., Zhang L.F., Zhang X., Li S.M., Xue F.Q. Tracing antibacterial compounds from Acalypha australis Linn. by spectrum-effect relationships and semi-preparative HPLC. J. Sep. Sci. 2013;36:1667–1676. doi: 10.1002/jssc.201201202. [DOI] [PubMed] [Google Scholar]
- 24.Mao X.L., Qin Y., Cai J., Zheng J.M., Ye Y.H., Liu H.G., Huang Z.S. Infrared fingerprint of Zathoxylum nitidum and its effect on inhibition of tumor cell. J. Infrared Millim. 2013;32:91–96. doi: 10.3724/SP.J.1010.2013.00091. [DOI] [Google Scholar]
- 25.Luo P., Liu Y., Lv L.Y., Zhang Z.F. Spectrum-effect correlation analysis of traditional Tibetan medicine “Morina nepalensis” on nitric oxide production inhibition. Zhongguo Zhong Yao Za Zhi. 2013;38:2882–2885. [PubMed] [Google Scholar]
- 26.Ma P., Zhang X.Y., Xu L.J., Wang Z., Xiao P.G. Spectrum-effect relation between anti-HIV 1 activities and ultra-performance liquid chromatography fingerprints of Rheum species. Zhongguo Zhong Yao Za Zhi. 2013;38:2434–2437. [PubMed] [Google Scholar]
- 27.Sun L.Q., Ding X.P., Qi J., Yu H., He S.A., Zhang J., Ge H.X., Yu B.Y. Antioxidant anthocyanins screening through spectrum–effect relationships and DPPH-HPLC-DAD analysis on nine cultivars of introduced rabbiteye blueberry in China. Food Chem. 2012;132:759–765. doi: 10.1016/j.foodchem.2011.11.030. [DOI] [Google Scholar]
- 28.Kong W.J., Zhao Y.L., Xiao X.H., Wang J.B., Li H.B., Li Z.L., Jin C., Liu Y. Spectrum-effect relationships between ultra performance liquid chromatography fingerprints and anti-bacterial activities of Rhizoma coptidis. Anal. Chim. Acta. 2009;634:279–285. doi: 10.1016/j.aca.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 29.Kong W.J., Zhao Y.L., Shan L.M., Xiao X.H., Guo W.Y. Investigation on the spectrum-effect relationships of EtOAC extract from Radix Isatidis based on HPLC fingerprints and microcalorimetry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2008;871:109–114. doi: 10.1016/j.jchromb.2008.06.053. [DOI] [PubMed] [Google Scholar]
- 30.Sun Z.Y., Zhao Y.L., Liu T.T., Sun X.J., Li R.S., Zhang P., Xiao X.H. Spectrum-effect relationships between UPLC fingerprints and bioactivities of five Aconitum L. plants. Thermochim. Acta. 2013;558:61–66. doi: 10.1016/j.tca.2013.02.011. [DOI] [Google Scholar]
- 31.Zhang L., Li J., Chen H., Wang Y.L., Wang A.M., Huang Y. Study on fingerprint-pharmacology correlation of protective effect of Polygonum orientale on myocardial cell oxidative injury induced by H2O2. Zhongguo Zhong Yao Za Zhi. 2012;37:2585–2588. [PubMed] [Google Scholar]
- 32.Bao Y.R., Wang S., Meng X.S., Yang X.X., Cui Y.L. Establishment of spectrum-effect relationship network model of Qizhiweitong granules promoting gastrointestinal motility. Zhong Yao Cai. 2014;37:828–832. [PubMed] [Google Scholar]
- 33.Kong W.J., Zhao Y.L., Shan L.M., Xiao X.H., Guo W.Y. Spectrum-effect relationships between HPLC fingerprints and biothermo-logical activity of Zuojinwan and its similar formulas. Acta Chem. Sin. 2008;66:2533–2538. [Google Scholar]
- 34.Li Y.J., Bi K.S. Study on the therapeutic material basis of traditional Chinese medicinal preparation suanzaoren decoction. Chem. Pharm. Bull. (Tokyo) 2006;54:847–851. doi: 10.1248/cpb.54.847. [DOI] [PubMed] [Google Scholar]
- 35.Chen Y., Yu H., Wu H., Pan Y., Wang K., Liu L., Jin Y., Zhang C. A novel reduplicate strategy for tracing hemostatic compounds from heating products of the flavonoid extract in platycladi cacumen by spectrum-effect relationships and column chromatography. Molecules. 2015;20:16970–16986. doi: 10.3390/molecules200916970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen X., Liu X., Chen Y., Hong Y., Feng S. Spectrum-effect relationship on anti-hepatic fibrosis effect of Radix Hedysari. Se Pu. 2015;33:413–418. doi: 10.3724/sp.j.1123.2014.12035. [DOI] [PubMed] [Google Scholar]
- 37.Tong Y., Zhu X., Yan Y., Liu R., Gong F., Zhang L., Hu J., Fang L., Wang R., Wang P. The influence of different drying methods on constituents and antioxidant activity of saffron from China. Int. J. Anal. Chem. 2015;2015:953164. doi: 10.1155/2015/953164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen Y., Yu H., Wu H., Pan Y., Wang K., Liu L., Jin Y., Zhang C. Tracing novel hemostatic compounds from heating products of total flavonoids in Flos Sophorae by spectrum-effect relationships and column chromatography. J. Sep. Sci. 2015;38:1691–1699. doi: 10.1002/jssc.201500100. [DOI] [PubMed] [Google Scholar]
- 39.Zheng Q., Zhao Y., Wang J., Liu T., Zhang B., Gong M., Li J., Liu H., Han B., Zhang Y., et al. Spectrum-effect relationships between UPLC fingerprints and bioactivities of crude secondary roots of Aconitum carmichaelii Debeaux (Fuzi) and its three processed products on mitochondrial growth coupled with canonical correlation analysis. J. Ethnopharmacol. 2014;153:615–623. doi: 10.1016/j.jep.2014.03.011. [DOI] [PubMed] [Google Scholar]
- 40.Zang Q.C., Wang J.B., Kong W.J., Jin C., Ma Z.J., Chen J., Gong Q.F., Xiao X.H. Searching for the main anti-bacterial components in artificial Calculus bovis using UPLC and microcalorimetry coupled with multi-linear regression analysis. J. Sep. Sci. 2011;34:3330–3338. doi: 10.1002/jssc.201100500. [DOI] [PubMed] [Google Scholar]
- 41.Chen Y., Wu C.M., Dai R.J., Li L., Yu Y.H., Li Y., Meng W.W., Zhang L., Zhang Y., Deng Y.L. Combination of HPLC chromatogram and hypoglycemic effect identifies isoflavones as the principal active fraction of Belamcanda chinensis leaf extract in diabetes treatment. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2011;879:371–378. doi: 10.1016/j.jchromb.2010.12.022. [DOI] [PubMed] [Google Scholar]
- 42.Donno D., Beccaro G.L., Mellano M.G., Cerutti A.K., Canterino S., Bounous G. Effect of agronomic and environmental conditions on chemical composition of tree-species buds used for herbal preparations. Vegenos. 2012;25:21–29. [Google Scholar]
- 43.Liu X., Wang X.L., Wu L., Li H., Qin K.M., Cai H., Pei K., Liu T., Cai B.C. Investigation on the spectrum-effect relationships of Da-Huang-Fu-Zi- Tang in rats by UHPLC-ESI-Q-TOF-MS method. J. Ethnopharmacol. 2014;154:606–612. doi: 10.1016/j.jep.2014.04.027. [DOI] [PubMed] [Google Scholar]
- 44.Ganzera M. Quality control of herbal medicines by capillary electrophoresis: Potential, requirements and applications. Electrophoresis. 2008;29:3489–3503. doi: 10.1002/elps.200700901. [DOI] [PubMed] [Google Scholar]
- 45.Xiao X.H., Yan D., Yuan H.L., Wang J.B., Cheng J. Novel patterns of efficient components recognition and quality control for Chinese material medica based on constituent Knock-out/Knock-in. Zhong Cao Yao. 2009;40:1345–1348. [Google Scholar]
- 46.Yan D., Li J., Xiong Y., Zhang C., Luo J., Han Y., Wang R., Jin C., Qian H., Li J. Promotion of quality standard of herbal medicine by constituent removing and adding. Sci. Rep. 2014;4 doi: 10.1038/srep03668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li J.X. Master’s Thesis. University of Science and Technology Kunming; Kunming, China: 2013. A Quality Evaluation Strategy for Active Constituents Recognition and Quality Control of Traditional Chinese Medicine (Rhizoma coptidis) by Constituent Knock-out/Knock-in Strategy. [Google Scholar]
- 48.Jin J., Li Y., Kipletting Tanui E., Han L., Jia Y., Zhang L., Wang Y., Zhang X., Zhang Y. Fishing and knockout of bioactive compounds using a combination of high-speed counter-current chromatography (HSCCC) and preparative HPLC for evaluating the holistic efficacy and interaction of the components of Herba Epimedii. J. Ethnopharmacol. 2013;147:357–365. doi: 10.1016/j.jep.2013.03.018. [DOI] [PubMed] [Google Scholar]
- 49.Yu L.Y., Wang Y., Fan X.H., Qu H.B., Cheng J.Y. Discovering active components from traditional Chinese medicine by component-Knock out approach. Zhongguo Zhong Yao Za Zhi. 2009;34:336–339. [PubMed] [Google Scholar]
- 50.Yan C.X. Master’s Thesis. University of Science and Technology Kunming; Kunming, China: 2013. Study on the Model for Efficient Component Recognition of Calculus Bovis Based on Target Constituents “Knock-out & Knock-in”. [Google Scholar]
- 51.Kong W.J. Ph.D. Thesis. Chengdu University of Traditional Chinese Medicine; Chengdu, China: 2011. A New Pattern for Efficient Constituents Recognition and Quality Controlof Traditional Chinese Drug (Calculus bovis) by Component “Knock-out/Knock-in” strategy. [Google Scholar]
- 52.Zhang T.T. Master’s Thesis. Chengdu University of Traditional Chinese Medicine; Chengdu, China: 2011. Novel Pattern of Efficient Components Recognition and Quality Control for Flos Lonicerae Japonicae Based on Constituent Knock-out/Knock-in. [Google Scholar]
- 53.Li X.F. Master’s Thesis. Hunan University of Traditional Chinese Medicine; Hunan, China: 2011. Novel Pattern of Efficient Components Recognition and Quality Control for Curcuma longa L. Based on Constituent Knock-out/Knock-in. [Google Scholar]
- 54.He J. Master’s Thesis. Kunming University of Science and Technology; Kunming, China: 2011. The Anti-Oxidative Efficient Component Recognition for Curcuma longa L. Based on the Quality Control Pattern of Constituent Knock-out/Knock-in. [Google Scholar]
- 55.Li S.M., Tan R., Gu J., Zeng H.S., Xiao X.H. The anti-atherosclerosis efficient component recognition for radix puerariae based on the quality control pattern of constituent Knock out. Ning Xia Yi Ke Da Xue Xue Bao. 2011;33:104. [Google Scholar]
- 56.Sheiner L.B., Stanski D.R., Vozeh S., Miller R.D., Ham J. Simultaneous modeling of pharmacokinetics and pharmacodynamics: Application to d-tubocurarine. Clin. Pharmacol. Ther. 1979;25:358–371. doi: 10.1097/00132586-198002000-00012. [DOI] [PubMed] [Google Scholar]
- 57.Peng L. Master’s Thesis. Beijing University of Chinese Medicine; Beijing, China: 2014. Study on the PK-PD Characteristics with Antipyretic Effect Following Intravenous Administration of Qingkailing Injection. [Google Scholar]
- 58.Wang Y.Q., Yan J.Y., Li S.X., Luo K., Peng M.J., Xie Y., Xu F. Study on pharmacokinetics-pharmacodynamics correlation of Yin Teng Gu Bi Kang prescription. Zhong Yao Cai. 2014;37:473–477. [PubMed] [Google Scholar]
- 59.Zhan S.Y., Shao Q., Li Z., Wang Y., Fan X.H. Study on PK-PD characteristics of ginsenoside Rg1 and Rb1 in rats with myocardial ischemia following intravenous administration of Shengmai injection. Zhongguo Zhong Yao Za Zhi. 2014;39:1300–1305. [PubMed] [Google Scholar]
- 60.Yan L. Master’s Thesis. Qufu Normal University; Qufu, China: 2014. Study on the PK-PD Characteristics of Curculigo in Rats. [Google Scholar]
- 61.Wang B.L., Hu J.P., Sheng L., Chen H., Li Y. Chemical-pharmacokinetic (PK)-pharmacodynamic (PD) fingerprints study of schisandra chinensis alcoholic extraction. Yao Xue Xue Bao. 2013;48:734–740. [PubMed] [Google Scholar]
- 62.Yang Y.M. Master’s Thesis. Chengdu University of Traditional Chinese Medicine; Chengdu, China: 2011. Pharmacokinetic-Pharmacodynamic Model of the Rhubarb Anthraquinone Treat on Intestinal Barrier Injury. [Google Scholar]
- 63.Li Q.S. Master’s Thesis. Dalian Medical University; Dalian, China: 2010. Multicomponent Pharmacokinetics and Antioxidation Pharmacodynamics of Tea Polyphenols in Rats as well as Their Correlations. [Google Scholar]
- 64.Hao H.P., Zhang C.N., Wang G.J. Thoughts and experimental exploration on pharmacokinetic study of herbal medicines with multiple-components and targets. Yao Xue Xue Bao. 2009;44:270–275. [PubMed] [Google Scholar]
- 65.Dong L.C., Zhang X.H., Ma J., Luo N., Song W., Li P., Li H.J. The integrated pharmacokinetics of major rhodojaponins correlates with the cardiotoxicity after oral administration of Rhododendri Mollis Flos extract in rats. J. Ethnopharmacol. 2014;157:69–78. doi: 10.1016/j.jep.2014.09.021. [DOI] [PubMed] [Google Scholar]
- 66.Guo Q., Li P., Wang Z., Cheng Y., Wu H., Yang B., Du S., Lu Y. Brain distribution pharmacokinetics and integrated pharmacokinetics of Panax Notoginsenoside R1, Ginsenosides Rg1, Rb1, Re and Rd in rats after intranasal administration of Panax Notoginseng Saponins assessed by UPLC/MS/MS. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2014;969:264–271. doi: 10.1016/j.jchromb.2014.08.034. [DOI] [PubMed] [Google Scholar]
- 67.Li X.Y., Hao H.P., Wang G.J., Guo S.J., Yan L., Lin X. Integrated pharmacokinetic study of multiple effective components contained in total Panax Notoginsenosides. Chin. J. Nat. Med. 2008;6:377–381. doi: 10.3724/SP.J.1009.2008.00377. [DOI] [Google Scholar]
- 68.Pan L., Zhou J., Zhu H., Wang W., Zhang M., Tian X., Lu J., Zeng M. Study on integrated pharmacokinetics of gardenia acid and geniposide: Time-antioxidant efficacy after oral administration of Huanglian-Zhizi couplet medicine from Huang-Lian-Jie-Du-Tang in MCAO rats. Am. J. Chin. Med. 2014;42:393–407. doi: 10.1142/S0192415X14500268. [DOI] [PubMed] [Google Scholar]
- 69.Zhu H., Qian Z., Li H., Guo L., Pan L., Zhang Q., Tang Y. Integrated pharmacokinetics of major bioactive components in MCAO rats after oral administration of Huang-Lian-Jie-Du-Tang. J. Ethnopharmacol. 2012;141:158–169. doi: 10.1016/j.jep.2012.02.014. [DOI] [PubMed] [Google Scholar]
- 70.Xie Y., Hao H., Kang A., Liang Y., Xie T., Sun S., Dai C., Zheng X., Xie L., Li J., et al. Integral pharmacokinetics of multiple lignan components in normal, CCl4-induced hepatic injury and hepatoprotective agents pretreated rats and correlations with hepatic injury biomarkers. J. Ethnopharmacol. 2010;131:290–299. doi: 10.1016/j.jep.2010.06.038. [DOI] [PubMed] [Google Scholar]
- 71.He W., Liu G., Cai H., Sun X., Hou W., Zhang P., Xie Z., Liao Q. Integrated pharmacokinetics of five protoberberine-typealkaloidsin normal and insomnic rats after single and multiple oral administration of Jiao-Tai-Wan. J. Ethnopharmacol. 2014;154:635–644. doi: 10.1016/j.jep.2014.04.040. [DOI] [PubMed] [Google Scholar]
- 72.Ma Z.T., Yang X.W., Zhang Y., Liu J.X. Pharmacochemistry and integrated pharmacokinetics of sixalkaloidsafter oral administration of huang-lian-jie-du-tang decoction. J. Asian Nat. Prod. Res. 2014;16:483–496. doi: 10.1080/10286020.2014.913577. [DOI] [PubMed] [Google Scholar]
- 73.Shi X.Y., Zhang F.L., Liang S., Koomson E., Edmond S., He X. Integral pharmacokinetic study of multiple components in total coumarins in Radix angelicae dahuricae; Proceedings of the 10th Conference on Drugs and Xenobiotics Metabolism of the Chinese Pharmacological Society; Nanjing, China. 21 September 2012. [Google Scholar]
- 74.Li Q.S., Xi H., Han G.Z., Wang C.Y., Lü L., Zou L.L., Li N. Integrated pharmacokinetic study of multiple effective components of tea polyphenols and its correlation with anti-free radical pharmacodynamics in rats. Yao Xue Xue Bao. 2012;47:863–869. [PubMed] [Google Scholar]
- 75.Zhang Q.Y., Xu L.H., Li B.T., Luo H., Tang X.L., Xu G.L. Classified and integrated pharmacokinetic study of multiple effective components contained in Gegen-Qinlian decoction. Zhong Guo Lin Chuang Yao Li Xue Yu Zhi Liao Xue. 2011;16:51–56. [Google Scholar]
- 76.Löbenberg R., Amidon G.L. Modern bioavailability, bioequivalence and biopharmaceutics classification system. New scientific approaches to international regulatory standards. Eur. J. Pharm. Biopharm. 2000;50:3–12. doi: 10.1016/S0939-6411(00)00091-6. [DOI] [PubMed] [Google Scholar]
- 77.Yu H., Zhao X., Zu Y., Zhang X., Zu B., Zhang X. Preparation and Characterization of Micronized Artemisinin via a Rapid Expansion of Supercritical Solutions (RESS) Method. Int. J. Mol. Sci. 2012;13:5060–5073. doi: 10.3390/ijms13045060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.He S., Lei Z.J., Zhang S.Y., Zhang Z.Y. Micronization of magnolia bark extract by RESS as well as dissolution and pharmacokinetics evaluation. Yao Xue Xue Bao. 2009;44:532–539. [PubMed] [Google Scholar]
- 79.Yen F.L., Wu T.H., Lin L.T., Cham T.M., Lin C.C. Nanoparticles formulation of Cuscuta chinensis prevents acetaminophen-induced hepatotoxicity in rats. Food Chem. Toxicol. 2008;46:1771–1777. doi: 10.1016/j.fct.2008.01.021. [DOI] [PubMed] [Google Scholar]
- 80.Yue P.F., Wan J., Wang Y., Li Y., Ma Y.Q., Yang M., Hu P.Y., Yuan H.L., Wang C.H. d-α-tocopherol acid polyethylene glycol 1000 succinate, an effective stabilizer during solidification transformation of baicalin nanosuspensions. Int. J. Pharm. 2013;443:279–287. doi: 10.1016/j.ijpharm.2012.12.036. [DOI] [PubMed] [Google Scholar]
- 81.Yue P.F., Li Y., Wan J. Study on formability of solid nanosuspensions during nanodispersion and solidification: I. Novel role of stabilizer/drug property. Int. J. Pharm. 2013;454:269–277. doi: 10.1016/j.ijpharm.2013.06.050. [DOI] [PubMed] [Google Scholar]
- 82.Hong C., Dang Y., Lin G., Yao Y., Li G., Ji G., Shen H., Xie Y. Effects of stabilizing agents on the development of myricetin nanosuspensiion and its characterization: An in vitro and in vivo evaluation. Int. J. Pharm. 2014;477:251–260. doi: 10.1016/j.ijpharm.2014.10.044. [DOI] [PubMed] [Google Scholar]
- 83.Han M., Yu X., Guo Y., Wang Y., Kuang H., Wang X. Honokiolnanosuspensions: Preparation, increased oral bioavailability and dramatically enhanced biodistribution in thecardio-cerebro-vascularsystem. Colloids Surf. B Biointerfaces. 2014;116:114–120. doi: 10.1016/j.colsurfb.2013.12.056. [DOI] [PubMed] [Google Scholar]
- 84.Lam P.L., Kok S.H., Bian Z.X., Lam K.H., Tang J.C., Lee K.K., Gambari R., Chui C.H. d-Glucose as a modifying agent in gelatin/collagen matrix and reservoir nanoparticles for Calendula officinalis delivery. Colloids Surf. B Biointerfaces. 2014;117:227–283. doi: 10.1016/j.colsurfb.2014.02.041. [DOI] [PubMed] [Google Scholar]
- 85.Jin S.-Y., Han J., Jin S.-X., Lv Q.-Y., Bai J.-X., Chen H.G., Li R.-S., Wu W., Yuan H.-L. Characterization and evaluation in vivo of baicalin-nanocrystals prepared by an ultrasonic-homogenization-fluridbed drying method. Chin. J. Nat. Med. 2014;12:71–80. doi: 10.1016/S1875-5364(14)60012-1. [DOI] [PubMed] [Google Scholar]
- 86.Jin S.Y., Yuan H.L., Jin S.X., Lv Q.Y., Bai J.X., Han J. Preparation of baicalin nanocrystal pellets and preliminary study on its pharmacokinetics. Zhongguo Zhong Yao Za Zhi. 2013;38:1156–1159. [PubMed] [Google Scholar]
- 87.Li Y., Wang Y., Yue P.F., Hu P.Y., Wu Z.F., Yang M., Yuan H.L. A novel high-pressure precipitation tandem homogenization technology for drug nanocrystals production-acase study with ursodeoxycholic acid. Pharm. Dev. Technol. 2014;19:662–670. doi: 10.3109/10837450.2013.819015. [DOI] [PubMed] [Google Scholar]
- 88.Yang X., Miao X., Cao F., Li S., Ai N., Chang Q., Lee S.M., Zheng Y. Nanosuspension development of scutellarein as an active and rapid orally absorbed precursor of its BCS class IV glycoside scutellarin. J. Pharm. Sci. 2014;103:3576–3584. doi: 10.1002/jps.24149. [DOI] [PubMed] [Google Scholar]
- 89.Li Y., Sun S., Chang Q., Zhang L., Wang G., Chen W., Miao X., Zheng Y. A strategy for the improvement of the bioavailability and antiosteoporosis activity of BCS IV flavonoid glycosides through the formulation of their lipophilic aglycone into nanocrystals. Mol. Pharm. 2013;10:2534–2542. doi: 10.1021/mp300688t. [DOI] [PubMed] [Google Scholar]
- 90.Zhao X., Wang G., Zhang B., Li H., Nie Q., Zang C., Zhao X. Development of silymarin nanocrystals lyophilized power applying nanosuspension technology. Zhongguo Zhong Yao Za Zhi. 2009;34:1503–1508. [PubMed] [Google Scholar]
- 91.Wang W., Kang Q., Liu N., Zhang Q., Zhang Y., Li H., Zhao B., Chen Y., Lan Y., Ma Q., et al. Enhanced dissolution rate and oral bioavailability of Ginkgo biloba extract by preparing solid dispersion via hot-melt extrusion. Fitoterapia. 2015;102:189–197. doi: 10.1016/j.fitote.2014.10.004. [DOI] [PubMed] [Google Scholar]
- 92.Zhang Z., Chen Y., Deng J., Jia X., Zhou J., Lv H. Solid dispersion of berberine-phospholipid complex/TPGS 1000/SiO2: Preparation, characterization and in vivo studies. Int. J. Pharm. 2014;465:306–316. doi: 10.1016/j.ijpharm.2014.01.023. [DOI] [PubMed] [Google Scholar]
- 93.Yun F., Kang A., Shan J., Zhao X., Bi X., Li J., Di L. Preparation of osthole-polymersoliddispersions by hot-melt extrusion for dissolution and bioavailability enhancement. Int. J. Pharm. 2014;465:436–443. doi: 10.1016/j.ijpharm.2014.02.040. [DOI] [PubMed] [Google Scholar]
- 94.Zhao G., Duan J., Xie Y., Lin G., Luo H., Li G., Yuan X. Effects of solid dispersion and self-emulsifying formulations on the solubility, dissolution, permeability and pharmacokinetics of isorhamnetin, quercetin and kaempferol in total flavones of Hippophae rhamnoides L. Drug Dev. Ind. Pharm. 2013;39:1037–1045. doi: 10.3109/03639045.2012.699066. [DOI] [PubMed] [Google Scholar]
- 95.Xie Y., Li G., Yuan X., Cai Z., Rong R. Preparation and in vitro evaluation of solid dispersions of total flavones of Hippophae rhamnoides L. AAPS Pharm. Sci. Tech. 2009;10:631–640. doi: 10.1208/s12249-009-9246-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chen Y.L., Liao J.B., Liang Y.Z., Xie J.H., Wu Q., Lai X.P., Chen J.N., Su Z.R., Lin Z.X. Characterization of solid dispersions of Patchouli alcohol with different polymers: Effects on the inhibition of reprecipitation and the improvement of dissolution rate. Drug Dev. Ind. Pharm. 2015;41:436–444. doi: 10.3109/03639045.2013.877482. [DOI] [PubMed] [Google Scholar]
- 97.Yao L., Deng K.Y., Luo J.B. Preparation and in vitro dissolution of the solid dispersions of cinnamon oil. Nan Fang Yi Ke Da Xue Xue Bao. 2008;28:52–56. [PubMed] [Google Scholar]
- 98.Ding S.M., Zhang Z.H., Song J., Cheng X.D., Jiang J., Jia X.B. Enhanced bioavailability of apigenin viapreparation of a carbon nanopowder solid dispersion. Int. J. Nanomed. 2014;9:2327–2333. doi: 10.2147/IJN.S60938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Liu Q.Y., Zhang Z.H., Jin X., Jiang Y.R., Jia X.B. Enhanced dissolution and oral bioavailability of tanshinone IIA base by solid dispersion system with low-molecular-weight Chitosan. J. Pharm. Pharmacol. 2013;65:839–846. doi: 10.1111/jphp.12047. [DOI] [PubMed] [Google Scholar]
- 100.Jiang Y.R., Zhang Z.H., Huang S.Y., Lu Y., Ma T.T., Jia X.B. Enhanced dissolution and stability of Tanshinone IIA base bysoliddispersion system with nano-hydroxyapatite. Pharmacogn. Mag. 2014;10:332–337. doi: 10.4103/0973-1296.137375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Jiang Y.R., Zhang Z.H., Huang S.Y., Lu Y., Ma T.T., Jia X.B. An attempt to stabilize tanshinone IIA solid dispersion by the use of ternary systems with nano-CaCO3 and poloxamer 188. Pharmacogn. Mag. 2014;10(Suppl. 2):S311–S317. doi: 10.4103/0973-1296.133286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Jiang Y.R., Zhang Z.H., Ding D.M., Chen X.Y., Su E., Jia X.B. Comparison of different preparation methods of tanshinone-porous silica solid dispersion. Zhongguo Zhong Yao Za Zhi. 2013;38:3271–3276. [PubMed] [Google Scholar]
- 103.Jiang Y.R., Zhang Z.H., Xia H.J., Jia X.B. Study on solid dispersion of copovidone-based tanshinone IIA. Zhongguo Zhong Yao Za Zhi. 2013;38:174–178. [PubMed] [Google Scholar]
- 104.Jiang Y., Zhang Z., Lu Y., Tang J., Ma T., Jia X. Study on solid dispersion of binary vector of tanshinone IIA. Zhongguo Zhong Yao Za Zhi. 2012;37:1383–1387. [PubMed] [Google Scholar]
- 105.Wang C., Nie H., Li K., Zhang Y.X., Shu K.G., Chen X.J. Protective effect of baicalin solid dispersion on D-galactosamine induced acute hepatic injury in mice. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2014;34:71–74. [PubMed] [Google Scholar]
- 106.Cong W., Shen L., Xu D., Zhao L., Ruan K., Feng Y. Soliddispersion tablets of breviscapine with polyvinylpyrrolidone K30 for improved dissolution and bioavailability to commercial breviscapine tablets in beagle dogs. Eur. J. Drug Metab. Pharmacokinet. 2013;39:203–210. doi: 10.1007/s13318-013-0150-0. [DOI] [PubMed] [Google Scholar]
- 107.Hu S.Y., Zhang Z.H., Jia X.B. Study on andrographolide solid dispersion vectored by hydroxyapatite. Zhongguo Zhong Yao Za Zhi. 2013;38:341–345. [PubMed] [Google Scholar]
- 108.Hu S.Y., Zhang Z.H., Jiang Y.R., Ning Q., Liu Q.Y., Jia X.B. Studies on sustained release solid dispersion of tripterine carried by HPMC-stearic acid. Zhongguo Zhong Yao Za Zhi. 2012;37:3052–3055. [PubMed] [Google Scholar]
- 109.Yue P.F., Yuan H.L., Li X.Y., Yang M., Zhu W.F. Process optimization, characterization and evaluation in vivo of oxymatrine–phospholipid complex. Int. J. Pharm. 2010;387:139–146. doi: 10.1016/j.ijpharm.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 110.Jin X., Zhang Z.H., Sun E., Tan X.B., Zhu F.X., Jia X.B. A novel drug-phospholipid complex loaded micelle for baohuoside I enhanced oral absorption: In vivo and in vivo evaluations. Drug Dev. Ind. Pharm. 2013;39:1421–1430. doi: 10.3109/03639045.2012.719234. [DOI] [PubMed] [Google Scholar]
- 111.Jin X., Zhang Z.H., Sun E., Qian Q., Tan X.B., Jia X.B. Preparation of a nanoscale baohuoside I-phospholipid complex and determination of its absorption: In vivo and in vitro evaluations. Int. J. Nanomed. 2012;7:4907–4916. doi: 10.2147/IJN.S35965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wang H., Cui Y., Fu Q., Deng B., Li G., Yang J., Wu T., Xie Y. A phospholipid complex to improve the oral bioavailability of flavonoids. Drug Dev. Ind. Pharm. 2014;11:1–11. doi: 10.3109/03639045.2014.991402. [DOI] [PubMed] [Google Scholar]
- 113.Xia H.J., Zhang Z.H., Jin X., Hu Q., Chen X.Y., Jia X.B. A novel drug-phospholipid complex enriched with micelles: Preparation and evaluation in vitro and in vivo. Int. J. Nanomed. 2013;8:545–554. doi: 10.2147/IJN.S39526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zhao Y.Q., Wang L.P., Ma C., Zhao K., Liu Y., Feng N.P. Preparation and characterization of tetrandrine-phospholipid complex loaded lipid nanocapsules as potential oral carriers. Int. J. Nanomed. 2013;8:4169–4181. doi: 10.2147/IJN.S50557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zhou H., Wan J., Wu L., Yi T., Liu W., Xu H., Yang X. A New strategy for enhancing the oral bioavailability of drugs with poor water-solubility and low liposolubility based on phospholipid complex and supersaturated SEDDS. PLoS ONE. 2013;8:e84530. doi: 10.1371/journal.pone.0084530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Shi Y.J., Wu P.J., Wei P. Optimization on preparation of hawthorn fruit total flavonoids-phospholipid complex using Plackett-Burman design, central composite design and response surface methodology. Zhong Yao Cai. 2010;33:437–441. [PubMed] [Google Scholar]
- 117.Chen Z., Sun J., Liu D., Xiao Y., Cai B. Preparation of multivariant-phospholipid complex of Ginkgo biloba extract. Zhongguo Zhong Yao Za Zhi. 2010;35:2146–2150. [PubMed] [Google Scholar]
- 118.Jia D.S., Zhao J.L., Shi F., Jia X.B. Preparation of icaritin phytosomes and their solid dispersions. Zhong Cao Yao. 2010;41:1449–1453. [Google Scholar]
- 119.Jin X., Zhang Z.H., Sun E., Tan X.B., Zhu F.X., Li S.L., Jia X.B. Preparation of icariside II-phospholipid complexand its absorption across Caco-2 cell monolayers. Pharmazie. 2012;67:293–298. [PubMed] [Google Scholar]
- 120.Wu P.J., Xu R.C., Su Z.T., Wei P., Lin Y.J., Yang M., Zheng Q. The nasal mucosa permeability and toxicity of baicalin carrier systems liposomes, β-cyclodextrin inclusion compound, and phospholipid complex. Yao Xue Xue Bao. 2009;44:417–424. [PubMed] [Google Scholar]
- 121.Zhou Q., Wei X., Dou W., Chou G., Wang Z. Preparation and characterization of inclusion complexes formed between baicalein and cyclodextrins. Carbohydr. Polym. 2013;95:733–739. doi: 10.1016/j.carbpol.2013.02.038. [DOI] [PubMed] [Google Scholar]
- 122.Zhou Q., Zhong L., Wei X., Dou W., Chou G., Wang Z. Baicaleinand hydroxypropyl-γ-cyclodextrin complex in poloxamer thermal sensitive hydrogel for vaginal administration. Int. J. Pharm. 2013;454:125–134. doi: 10.1016/j.ijpharm.2013.07.006. [DOI] [PubMed] [Google Scholar]
- 123.Hsu C.M., Yu S.C., Tsai F.J., Tsai Y. Enhancement of rhubarb extract solubility and bioactivity by 2-hydroxypropyl-β-cyclodextrin. Carbohydr. Polym. 2013;98:1422–1429. doi: 10.1016/j.carbpol.2013.07.029. [DOI] [PubMed] [Google Scholar]
- 124.Yao Y., Xie Y., Hong C., Li G., Shen H., Ji G. Dvelopment myricetin/hydroxypropyl-β-cyclodextrin inclusion complex: Preparation, characterization, and evaluation. Carbohydr. Polym. 2014;110:329–337. doi: 10.1016/j.carbpol.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 125.Tsao J.Y., Tsai H.H., Wu C.P., Lin P.Y., Su S.Y., Chen L.D., Tsai F.J., Tsai Y. Release of paeonol-β-CD complex from thermo-sensitivepoly(N-isopropylacrylamide) hydrogels. Int. J. Pharm. 2010;402:123–128. doi: 10.1016/j.ijpharm.2010.09.033. [DOI] [PubMed] [Google Scholar]
- 126.Lu Y., Zhang T., Tao J., Ji G., Wang S. Preparation, characterization, and pharmacokinetics of the inclusion complex of genipin-β-cyclodextrin. Drug Dev. Ind. Pharm. 2009;35:1452–1459. doi: 10.3109/03639040903002151. [DOI] [PubMed] [Google Scholar]
- 127.Cui L., Zhang Z.H., Sun E., Jia X.B. Effect of β-cyclodextrin complexation on solubility and enzymatic conversion of naringin. Int. J. Mol. Sci. 2012;13:14251–14261. doi: 10.3390/ijms131114251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zhang Y., Wang Q.S., Cui Y.L., Meng F.C., Lin K.M. Changes in the intestinal absorption mechanism of icariin in the nanocavities of cyclodextrins. Int. J. Nanomed. 2012;7:4239–4249. doi: 10.2147/IJN.S33014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Cui L., Zhang Z., Sun E., Jia X., Qian Q. Effect of β-cyclodextrin complexation on solubility and enzymatic hydrolysis rate of icariin. J. Nat. Sci. Biol. Med. 2013;4:201–206. doi: 10.4103/0976-9668.107291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zhang Y., Meng F.C., Cui Y.L., Song Y.F. Enhancing effect of hydroxypropyl-β-Cyclodextrin ontheintestinal absorption process of genipin. J. Agric. Food Chem. 2011;59:10919–10926. doi: 10.1021/jf202712y. [DOI] [PubMed] [Google Scholar]
- 131.Liu C., Zhang W., Yang H., Sun W., Gong X., Zhao J., Sun Y., Diao G. A water-soluble inclusion complex of pedunculoside with the polymer β-cyclodextrin: A novel anti-inflammation agent with low toxicity. PLoS ONE. 2014;9:e101761. doi: 10.1371/journal.pone.0101761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Liu C., Zhang W., Yang H., Sun W., Gong X., Zhao J., Sun Y., Diao G. Preparation and evaluation of andrographolide-loaded microemulsion. J. Microencapsul. 2012;29:657–665. doi: 10.3109/02652048.2012.680508. [DOI] [PubMed] [Google Scholar]
- 133.Wu H., Lu C., Zhou A., Min Z., Zhang Y. Enhanced oral bioavailability of puerarin using microemulsion vehicle. Drug Dev. Ind. Pharm. 2009;35:138–144. doi: 10.1080/03639040801973495. [DOI] [PubMed] [Google Scholar]
- 134.Tang T.T., Hu X.B., Liao D.H., Liu X.Y., Xiang D.X. Mechanisms of microemulsion enhancing the oral bioavailability of puerarin: Comparison between oil-in-water and water-in-oil microemulsions using the single-pass intestinal perfusion method and a chylomicron flow blocking approach. Int. J. Nanomed. 2013;8:4415–4426. doi: 10.2147/IJN.S51469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zhao J.H., Ji L., Wang H., Chen Z.Q., Zhang Y.T., Liu Y., Feng N.P. Microemulsion-based novel transdermal delivery of tetramethylpyrazine: Preparation and evaluation in vitro and in vivo. Int. J. Nanomed. 2011;6:1611–1619. doi: 10.2147/IJN.S23597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zhang Y.T., Zhao J.H., Zhang S.J., Zhong Y.Z., Wang Z., Liu Y., Shi F., Feng N.P. Enhanced transdermal delivery of evodiamine and rutaecarpine using microemulsion. Int. J. Nanomed. 2011;6:2469–2482. doi: 10.2147/IJN.S25258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Qu D., He J., Liu C., Zhou J., Chen Y. Triterpene-loaded microemulsion using Coix lacyma-jobi seed extract as oil phase for enhanced antitumor efficacy: Preparation and in vivo evaluation. Int. J. Nanomed. 2014;9:109–119. doi: 10.2147/IJN.S54796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Shen L.N., Zhang Y.T., Wang Q., Xu L., Feng N.P. Preparation and evaluation of microemulsion-based transdermal delivery of total flavones of rhizome arisaematis. Int. J. Nanomed. 2014;9:3453–3464. doi: 10.2147/IJN.S66524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wen R., Li H., Du S., Zhao X., Zhao Z., Bai J., Lu Y. Preparation of Mpeg2000-PLA-modified Xingnaojing microemulsion and evaluation in mucosal irritation. J. Biomater. Sci. Polym. Ed. 2014;25:923–942. doi: 10.1080/09205063.2014.913467. [DOI] [PubMed] [Google Scholar]
- 140.He J.J., Chen Y., Du M., Cao W., Yuan L., Zheng L.Y. Exploration of one-step preparation of Ganoderma lucidum multicomponent microemulsion. Yao Xue Xue Bao. 2013;48:441–446. [PubMed] [Google Scholar]
- 141.Chen Y., Lu H., Song S., Jia X. Preparation of Ganoderma lucidum polysaccharides and triterpenes microemulsion and its anticancer effect in mice with transplant Heps tumors. Zhongguo Zhong Yao Za Zhi. 2010;35:2679–2683. [PubMed] [Google Scholar]
- 142.Liu J.Y., Han Y., Hu J.H., Wang Z.T., Chen K.X. The preparation of paeonol transdermal delivery systems based on the microemulsion-based gels and its pharmacokinetics characters. Yao Xue Xue Bao. 2012;47:244–249. [PubMed] [Google Scholar]
- 143.Wang L., Guo Q., Zhang Y., Shi Z. Preparation of Xiongbing microemulsion and its quality evaluation. Zhongguo Zhong Yao Za Zhi. 2011;36:142–146. [PubMed] [Google Scholar]
- 144.Gui S., Wu L., Pan J., Wen Z., Kai W., Wang J. Study on preparation of berberine microemulsion and its absorption in intestine. Zhongguo Zhong Yao Za Zhi. 2009;34:398–401. [PubMed] [Google Scholar]
- 145.Gui S.Y., Wu L., Peng D.Y., Liu Q.Y., Yin B.P., Shen J.Z. Preparation and evaluation of a microemulsion for oral delivery of berberine. Pharmazie. 2008;63:516–519. [PubMed] [Google Scholar]
- 146.Lü F.Q., Li H., Xu W., Zhang X., Huang M.Q., Zheng J., Chu K.D. Preparation of self-microemulsion drug delivery system of the mixture of paeonol and borneol based on Xingbi Fang. Yao Xue Xue Bao. 2013;48:1602–1610. [PubMed] [Google Scholar]
- 147.Xuan X.Y., Wang Y.J., Tian H., Pi J.X., Sun S.Z., Zhang W.L. Study on prescription of self-microemulsifying drug delivery system of Mangiferin phospholipid complex. Zhong Yao Cai. 2012;35:1508–1511. [PubMed] [Google Scholar]
- 148.Xie Y., Rong R., Li G., Yuan X., Wang J. Studies on self-microemulsifying drug preparations of total flavones of Hippophae rhamnoides. Zhongguo Zhong Yao Za Zhi. 2009;34:43–46. [PubMed] [Google Scholar]
- 149.Zhang J., Li Y., Gao W., Repka M.A., Wang Y., Chen M. Andrographolide-loaded PLGA-PEG-PLGA micelles to improve its bioavailability and anticancer efficacy. Expert Opin. Drug Deliv. 2014;11:1367–1380. doi: 10.1517/17425247.2014.924503. [DOI] [PubMed] [Google Scholar]
- 150.Li H., Wen X.S., Di W. In vitro and in vivo evaluation of Triptolide-loaded pluronic P105 polymeric micelles. Arzneimittelforschung. 2012;62:340–344. doi: 10.1055/s-0032-1312602. [DOI] [PubMed] [Google Scholar]
- 151.Gao H., Fan Y., Wang D., Hu Y., Liu J., Zhao X., Guo L., Zhao X., Yuan J., Zhang F. Optimization on preparation condition of epimedium polysaccharide lipsome and evaluation of its adjuvantactivity. Int. J. Biol. Macromol. 2012;50:207–213. doi: 10.1016/j.ijbiomac.2011.10.021. [DOI] [PubMed] [Google Scholar]
- 152.Gao H., Gao Q., Wang D.Y., Fan Y.P., Guo L.W., Zhao X.N. Preparation conditions optimization of Epimedium polysaccharide liposome. Zhong Yao Cai. 2011;34:1429–1433. [PubMed] [Google Scholar]
- 153.Zhao X., Liu J., Hu Y., Fan Y., Wang D., Yuan J., Xu L., Cui L., Jing Z. Optimization on condition of glycyrrhetinic acid liposome by RSM and the research of its immunological activity. Int. J. Biol. Macromol. 2012;51:299–304. doi: 10.1016/j.ijbiomac.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 154.Fan Y., Liu J., Wang D., Song X., Hu Y., Zhang C., Zhao X., Nguyen T.L. The preparation optimization and immune effect of epimedium polysaccharide-propolis flavone liposome. Carbohydr. Polym. 2013;94:24–30. doi: 10.1016/j.carbpol.2012.12.071. [DOI] [PubMed] [Google Scholar]
- 155.Huang Y., Wu C., Liu Z., Hu Y., Shi C., Yu Y., Zhao X., Liu C., Liu J., Wu Y. Optimization on preparation conditions of Rehmannia glutinosa polysaccharide liposome and its immunological activity. Carbohydr. Polym. 2014;104:118–126. doi: 10.1016/j.carbpol.2014.01.022. [DOI] [PubMed] [Google Scholar]
- 156.Wu R.G., Dai J.D., Wu F.G., Zhang X.H., Li W.H., Wang Y.R. Competitive molecular interaction among paeonol-loaded liposomes: Differential scanning calorimetry andsynchrotron X-ray diffraction studies. Int. J. Pharm. 2012;438:91–97. doi: 10.1016/j.ijpharm.2012.08.052. [DOI] [PubMed] [Google Scholar]
- 157.Shi J., Ma F., Wang X., Wang F., Liao H. Formulation of liposomes gels of paeonol for transdermal drug delivery by Box-Behnken statistical design. J. Liposome Res. 2012;22:270–278. doi: 10.3109/08982104.2012.690159. [DOI] [PubMed] [Google Scholar]
- 158.Yu Y., Lu Y., Bo R., Huang Y., Hu Y., Liu J., Wu Y., Tao Y., Wang D. The preparation of gypenosides liposomes and its effects on the peritoneal macrophages function in vitro. Int. J. Pharm. 2014;460:248–254. doi: 10.1016/j.ijpharm.2013.11.018. [DOI] [PubMed] [Google Scholar]
- 159.Yu Y., Lu Y., Bo R., Huang Y., Hu Y., Liu J., Wu Y., Tao Y., Wang D. Development of Salvianolic acid B-Tanshinone II A-Glycyrrhetinic acid compound liposomes: Formulation optimization and its effects on proliferation of hepatic stellate cells. Int. J. Pharm. 2014;462:11–18. doi: 10.1016/j.ijpharm.2013.12.040. [DOI] [PubMed] [Google Scholar]
- 160.Liu D., Hu H., Lin Z., Chen D., Zhu Y., Hou S., Shi X. Quercetin deformable liposome: Preparation and efficacy against ultraviolet B induced skin damages in vitro and in vivo. J. Photochem. Photobiol. B. 2013;127:8–17. doi: 10.1016/j.jphotobiol.2013.07.014. [DOI] [PubMed] [Google Scholar]
- 161.Tao Y., Wang D., Hu Y., Huang Y., Yu Y., Wang D. The immunological enhancement activity of propolis flavonoids liposome in vitro and in vivo. Evid. Based Complement. Alternat. Med. 2014;2014:483513. doi: 10.1155/2014/483513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Yuan J., Lu Y., Abula S., Hu Y., Liu J., Fan Y., Zhao X., Wang D., Liu X., Liu C. Optimization on preparation condition of propolis flavonoids liposome by response surface methodology and research of its immune enhancement activity. Evid. Based Complement. Alternat. Med. 2013;2013:505703. doi: 10.1155/2013/505703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Chen J., He C.Q., Lin A.H., Xu F., Wang F., Zhao B., Liu X., Chen Z.P., Cai B.C. Brucine-loaded liposomes composed of HSPC and DPPC at different ratios: In vitro and in vivo evaluation. Drug Dev. Ind. Pharm. 2014;40:244–251. doi: 10.3109/03639045.2012.756009. [DOI] [PubMed] [Google Scholar]
- 164.Chen J., Yan G.J., Hu R.R., Gu Q.W., Chen M.L., Gu W., Chen Z.P., Cai B.C. Improved pharmacokinetics and reduced toxicity of brucine after encapsulation into stealth liposomes: Role of phosphatidylcholine. Int. J. Nanomed. 2012;7:3567–3577. doi: 10.2147/IJN.S32860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Zhou Y., Ning Q., Yu D.N., Li W.G., Deng J. Improved oral bioavailability of breviscapine via a Pluronic P85-modified liposomal delivery system. J. Pharm. Pharmacol. 2014;66:903–911. doi: 10.1111/jphp.12215. [DOI] [PubMed] [Google Scholar]
- 166.Zhang Y.T., Shen L.N., Wu Z.H., Zhao J.H., Feng N.P. Evaluation of skin viability effect on ethosome and liposome-mediated psoralen delivery via cell uptake. J. Pharm. Sci. 2014;103:3120–3126. doi: 10.1002/jps.24096. [DOI] [PubMed] [Google Scholar]
- 167.Lin C.H., Al-Suwayeh S.A., Hung C.F., Chen C.C., Fang J.Y. Camptothecin-loaded liposomes with α-melanocyte-stimulating hormone enhnace cytotoxicity toward and cellular uptake by melanomas: An application of nanomedicine on natural product. J. Tradit. Complement. Med. 2013;3:102–109. doi: 10.4103/2225-4110.110423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Song J., Shi F., Zhang Z., Zhu F., Xue J., Tan X., Zhang L., Jia X. Formulation and evaluation of celastrol-loaded liposomes. Molecules. 2011;16:7880–7892. doi: 10.3390/molecules16097880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Chen Y., Wu Q., Zhang Z., Yuan L., Liu X., Zhou L. Preparation of curcumin-loaded liposomes and evaluation of their skin permeation and pharmacodynamics. Molecules. 2012;17:5972–5987. doi: 10.3390/molecules17055972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.He C.Q., Hu M.Y., Zhang H., Chang H., Chen J., Cai B.C. Study on preparation and thermosensitive release property of composite phospholipid liposomes containing total alkaloids from Strychnos nux-vomica. Zhongguo Zhong Yao Za Zhi. 2013;38:1366–1370. [PubMed] [Google Scholar]
- 171.He C.Q., Hu M.Y., Zhang H., Chang H., Chen J., Cai B.C. Preparation of freeze-dried long-circulation oridonin liposomes and their pharmacokinetics in rats. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2013;42:638–643. doi: 10.3785/j.issn.1008-9292.2013.06.008. [DOI] [PubMed] [Google Scholar]
- 172.Wu M., Liu J., Zhang X. Preparation of nano-liposome enveloping Flos Magnoliae volatile oil. Zhong Xi Yi Jie He Xue Bao. 2007;5:314–317. doi: 10.3736/jcim20070316. [DOI] [PubMed] [Google Scholar]
- 173.Liu Y., Wang P., Sun C., Zhao J., Du Y., Shi F., Feng N. Bioadhesion and enhanced bioavailability by wheat germ agglutinin-grafted lipid nanoparticles for oral delivery of poorly water-soluble drug bufalin. Int. J. Pharm. 2011;419:260–265. doi: 10.1016/j.ijpharm.2011.07.019. [DOI] [PubMed] [Google Scholar]
- 174.Wang S., Chen T., Chen R., Hu Y., Chen M., Wang Y. Emod in loaded solid lipid nanoparticles: Preparation, characterization and antitumor activity studies. Int. J. Pharm. 2012;430:2012. doi: 10.1016/j.ijpharm.2012.03.027. [DOI] [PubMed] [Google Scholar]
- 175.Zhang W., Li X., Ye T., Chen F., Sun X., Kong J., Yang X., Pan W., Li S. Design, characterization, and in vitro cellular inhibition and uptake of optimized genistein-loaded NLC for the prevention of posterior capsular opacification using response surface methodology. Int. J. Pharm. 2013;454:354–366. doi: 10.1016/j.ijpharm.2013.07.032. [DOI] [PubMed] [Google Scholar]
- 176.Huang X., Chen Y.J., Peng D.Y., Li Q.L., Wang X.S., Wang D.L., Chen W.D. Solid lipid nanoparticles as delivery systems for Gambogenic acid. Colloids. Surf. B Biointerfaces. 2013;102:391–397. doi: 10.1016/j.colsurfb.2012.08.058. [DOI] [PubMed] [Google Scholar]
- 177.Sun J., Bi C., Chan H.M., Sun S., Zhang Q., Zheng Y. Curcumin-loaded solid lipid nanoparticles have prolonged in vitro antitumour activity, cellular uptake and improved in vivo bioavailability. Colloids. Surf. B Biointerfaces. 2013;111:367–375. doi: 10.1016/j.colsurfb.2013.06.032. [DOI] [PubMed] [Google Scholar]
- 178.Xue M., Zhao Y., Li X.J., Jiang Z.Z., Zhang L., Liu S.H., Li X.M., Zhang L.Y., Yang S.Y. Comparison of toxicokinetic and tissue distribution of triptolide-loaded solid lipid nanoparticles vs. free triptolide in rats. Eur. J. Pharm. Sci. 2012;47:713–717. doi: 10.1016/j.ejps.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 179.Zhang C., Peng F., Liu W., Wan J., Wan C., Xu H., Lam C.W., Yang X. Nanostructured lipid carriers as a novel oral delivery system for triptolide: Induced changes in pharmacokinetics profile associated with reduced toxicity in male rats. Int. J. Nanomed. 2014;9:1049–1063. doi: 10.2147/IJN.S55144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Liu Z., Zhang X., Wu H., Li J., Shu L., Liu R., Li L., Li N. Preparation and evaluation of solid lipid nanoparticles of baicalin for ocular drug delivery system in vitro and in vivo. Drug Dev. Ind. Pharm. 2011;37:475–481. doi: 10.3109/03639045.2010.522193. [DOI] [PubMed] [Google Scholar]
- 181.Wang L., Luo Q., Lin T., Li R., Zhu T., Zhou K., Ji Z., Song J., Jia B., Zhang C., et al. PEGylated nanostructured lipid carriers (PEG-NLC) as a novel drug delivery system for biochanin A. Drug Dev. Ind. Pharm. 2014;10:1–9. doi: 10.3109/03639045.2014.938082. [DOI] [PubMed] [Google Scholar]
- 182.Zhao X.L., Yang C.R., Yang K.L., Li K.X., Hu H.Y., Chen D.W. Preparation and characterization of nanostructured lipid carriers loaded traditional Chinese medicine, zedoary turmeric oil. Drug Dev. Ind. Pharm. 2010;36:773–780. doi: 10.3109/03639040903485716. [DOI] [PubMed] [Google Scholar]
- 183.Shi F., Zhao J.H., Liu Y., Wang Z., Zhang Y.T., Feng N.P. Preparation and characterization of solid lipid nanoparticles loaded with frankincense and myrrh oil. Int. J. Nanomed. 2012;7:2033–2043. doi: 10.2147/IJN.S30085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Chen Y., Zhou L., Yuan L., Zhang Z.H., Liu X., Wu Q. Formulation, characterization, and evaluation of in vitro skin permeation and in vivo pharmacodynamics of surface-charged tripterine-loaded nanostructured lipid carriers. Int. J. Nanomed. 2012;7:3023–3032. doi: 10.2147/IJN.S32476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Zhang K., Lv S., Li X., Feng Y., Li X., Liu L., Li S., Li Y. Preparation, characterization, and in vivo pharmacokinetics of nanostructured lipid carriers loaded with oleanolic acid and gentiopicrin. Int. J. Nanomed. 2013;8:3227–3239. doi: 10.2147/IJN.S45031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Zhang C.G., Zhu Q.L., Zhou Y., Liu Y., Chen W.L., Yuan Z.Q., Yang S.D., Zhou X.F., Zhu A.J., Zhang X.N. N-Succinyl-chitosan nanoparticles coupled with low-density lipoprotein for targeted osthole-loaded delivery to low-density lipoprotein receptor-rich tumors. Int. J. Nanomed. 2014;9:2919–2932. doi: 10.2147/IJN.S59799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Yang T., Sheng H.H., Feng N.P., Wei H., Wang Z.T., Wang C.H. Preparation of and rographolide-loaded solid lipid nanoparticles and their in vitro and in vivo evaluations: Characteristics, release, absorption, transports, pharmacokinetics, and antihyperlipidemic activity. J. Pharm. Sci. 2013;102:4414–4425. doi: 10.1002/jps.23758. [DOI] [PubMed] [Google Scholar]
- 188.Zhang S.J., Zhang Y.T., Zhao J.H., Shen L.N., Shi F., Feng N.P. Preparationand in vitro anti-tumor properties of toad venom extract-loaded solid lipid nanoparticles. Pharmazie. 2013;68:653–660. [PubMed] [Google Scholar]
- 189.Zhang X., Lü S., Han J., Sun S., Wang L., Li Y. Preparation, characterization and in vivo distribution of solid lipid nanoparticles loaded with syringopicroside. Pharmazie. 2011;66:404–407. [PubMed] [Google Scholar]
- 190.Zhang Y.L., Zhang Z.H., Jiang T.Y., Ayman-Waddad, Jing L., Lv H.X., Zhou J.P. Cell uptake of paclitaxel solid lipid nanoparticles modified by cell-penetrating peptides in A549 cells. Pharmazie. 2013;68:47–53. [PubMed] [Google Scholar]
- 191.Qi H., Li L., Huang C., Li W., Wu C. Optimization and physicochemical characterization of thermosensitive poloxamer gel containing puerarin for ophthalmic use. Chem. Pharm. Bull. (Tokyo) 2006;54:1500–1507. doi: 10.1248/cpb.54.1500. [DOI] [PubMed] [Google Scholar]
- 192.Zhou L., Chow M.S., Zuo Z. Effect of sodium caprate on the oral absorptions of danshensu and salvianolic acid B. Int. J. Pharm. 2009;379:109–118. doi: 10.1016/j.ijpharm.2009.06.016. [DOI] [PubMed] [Google Scholar]
- 193.Xiao B., Li Q., Han N., Zhang C.L., Yin J. Soft tissue contusion repairing effects of Hong Yao with different penetration enhancers. J. Ethnopharmacol. 2013;148:610–616. doi: 10.1016/j.jep.2013.05.012. [DOI] [PubMed] [Google Scholar]
- 194.Zhang C.F., Yang Z.L., Luo J.B., Zhu Q.H., Zhao H.N. Effects of cinnamene enhancers on transdermal delivery of ligustrazine hydrochloride. Eur. J. Pharm. Biopharm. 2007;67:413–419. doi: 10.1016/j.ejpb.2007.02.019. [DOI] [PubMed] [Google Scholar]
- 195.Gu S., Gao J., Hou X., Ding B., Zhang W., Gao S., Ding X. Effects of penetration enhancers on Shuangwu traumatic formula: In vitro percutaneous absorption and in vivo pharmacodynamic evaluation of an herb medicine. Eur. J. Pharm. Biopharm. 2009;73:385–390. doi: 10.1016/j.ejpb.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 196.Liu R., Liu Z., Zhang C., Zhang B. Gelucire44/14as a novel absorption enhancer for drugs with different hydrophilicities: In vitro and in vivo improvement on transcorneal permeation. J. Pharm. Sci. 2011;100:3186–3195. doi: 10.1002/jps.22540. [DOI] [PubMed] [Google Scholar]
- 197.Li Z., Sun D., Yang H., Liu X., Luan L., Bai J., Cui H. Effect of borneol on the distribution of danshensu to the eye in rabbit via oral administration. Curr. Eye Res. 2010;35:565–572. doi: 10.3109/02713681003718091. [DOI] [PubMed] [Google Scholar]
- 198.Xie Y., Luo H., Duan J., Hong C., Ma P., Li G., Zhang T., Wu T., Ji G. Phytic acid enhances the oral absorption of isorhamnetin, quercetin, and kaempferol in total favones of Hippophae rhamnoides L. Fitoterapia. 2014;93:216–225. doi: 10.1016/j.fitote.2014.01.013. [DOI] [PubMed] [Google Scholar]
- 199.Wang S., Zhu W., Ou S., Guan Y., Chen L., Yang M. Effects of penetration enhancers on in vitro percutaneous absorption and amount retained in skin of paeonol, dictamnine, fraxinellone and glycyrrhetinic acid in Liangfu cream. Zhongguo Zhong Yao Za Zhi. 2009;34:1778–1782. [PubMed] [Google Scholar]
- 200.Lin X., Lu Z., Xu D., Feng Y., Shen L. Oral absorption enhancers of Ophiopogon japonicas polysaccharides. Zhongguo Zhong Yao Za Zhi. 2009;34:1498–1502. [PubMed] [Google Scholar]
- 201.Luo M.F., Shen Q., Zhang T., Xu Y.H. Effect of Atractylodes Rhizome oil and other volatile oils on percutaneous absorption of baicalin. Zhong Yao Cai. 2008;31:1721–1724. [PubMed] [Google Scholar]
- 202.Sha M., Yin L.F., Xu W., Chen Y.Z. Effects of 2-N-nonyl-1,3-dioxolane as an enhancer on transdermal absorption of Salvia miltiorrhiza gel. Zhongguo Zhong Yao Za Zhi. 2007;32:487–489. [PubMed] [Google Scholar]
- 203.Zhang C.F., Yang Z.L., Luo J.B. Effects of d-limonene and l-limonene on transdermal absorption of ligustrazine hydrochloride. Yao Xue Xue Bao. 2006;41:772–777. [PubMed] [Google Scholar]
- 204.Zhou W., Zhu X.X., Yin A.L., Cai B.C., Wang H.D., Di L., Shan J.J. Effect of various absorption enhancers based on tight junctions on the intestinal absorption of forsythoside A in Shuang-Huang-Lian, application to its antivirus activity. Pharmacogn. Mag. 2014;10:9–17. doi: 10.4103/0973-1296.126651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Liu R., Liu Z., Shu L., Zhang C., Zhang B. Effect of three penetration enhancers on corneal permeability of mangiferin in vitro. Zhongguo Zhong Yao Za Zhi. 2010;35:3131–3135. [PubMed] [Google Scholar]
- 206.Shi Z.H., Xiong F.L., Huang Z.J., Xiong D.K., Zeng Q.H. Effects of penetration enhancers on percutaneous permeability of geniposide in Xiaoer Niuhuang tuire cataplasms. Zhongguo Zhong Yao Za Zhi. 2008;33:2061–2063. [PubMed] [Google Scholar]
- 207.China Pharmacopoeia Committee, Pharmacopoeia of People’s Republic of China. 9th ed. Volume 1 China Medical Science and Technology Press; Beijing, China: 2010. [Google Scholar]
- 208.Zhou W., Shan J.J., Ju W.Z., Wang S.C., Meng M.X., Cai B.C., Di L.Q. Simultaneous determination of twenty-six components of Flos Lonicerae Japonicae-Fructus Forsythiae herb couple using UPLC-ESI-MS/MS: Application to its preparations. Anal. Methods. 2015;7:1425–1437. doi: 10.1039/C4AY01477D. [DOI] [Google Scholar]
- 209.Zhou W., Tam K.Y., Meng M., Shan J., Wang S., Ju W., Cai B., Di L. Pharmacokinetics screening for multi-components absorbed in the rat plasma after oral administration of traditional Chinese medicine Flos Lonicerae Japonicae-Fructus Forsythiae herb couple by sequential negative and positive ionization ultra-high-performance liquid chromatography/tandem triple quadrupole mass spectrometric detection. J. Chromatogr. A. 2015;1376:84–97. doi: 10.1016/j.chroma.2014.12.018. [DOI] [PubMed] [Google Scholar]
- 210.Zhou W., Tan X., Shan J., Wang S., Yin A., Cai B., Di L. Study on the main components interaction from Flos Lonicerae and Fructus Forsythiae and their dissolution in vitro and intestinal absorption in rats. PLoS ONE. 2014;9:e109619. doi: 10.1371/journal.pone.0109619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Zhou W., Tan X., Shan J., Liu T., Cai B., Di L. Effect of chito-oligosaccharide on the intestinal absorptions of phenylethanoid glycosides in Fructus Forsythiae extract. Phytomedicine. 2014;21:1549–1558. doi: 10.1016/j.phymed.2014.06.016. [DOI] [PubMed] [Google Scholar]
- 212.Zhou W., Shan J., Tan X., Zou J., Yin A., Cai B., Di L. Effect of chito-oligosaccharide on the oral absorptions of phenolic acids of Flos Lonicerae extract. Phytomedicine. 2014;21:184–194. doi: 10.1016/j.phymed.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Zhou W., Qin K.M., Shan J.J., Ju W.Z., Liu S.J., Cai B.C., Di L.Q. Improvement of intestinal absorption of forsythoside A in weeping forsythia extract by various absorption enhancers based on tight junctions. Phytomedicine. 2012;20:47–58. doi: 10.1016/j.phymed.2012.09.014. [DOI] [PubMed] [Google Scholar]
- 214.Zhou W., Di L.Q., Wang J., Shan J.J., Liu S.J., Ju W.Z., Cai B.C. Intestinal absorption of forsythoside A in in situ single-pass intestinal perfusion and in vitro Caco-2 cell models. Acta Pharmacol. Sin. 2012;33:1069–1079. doi: 10.1038/aps.2012.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Gao Y., He L., Katsumi H., Sakane T., Fujita T., Yamamoto A. Improvement of intestinal absorption of insulin and water-soluble macromolecular compounds by chitosan oligomers in rats. Int. J. Pharm. 2008;359:70–78. doi: 10.1016/j.ijpharm.2008.03.016. [DOI] [PubMed] [Google Scholar]
- 216.Zhou W., Wang H., Zhu X., Shan J., Yin A., Cai B., Di L. Improvement of intestinal absorption of forsythoside A and chlorogenic acid by different carboxymethyl chitosan and chito-oligosaccharide, application to Flos Lonicerae-Fructus Forsythiae herb couple preparations. PLoS ONE. 2013;8:e63348. doi: 10.1371/journal.pone.0063348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Ma L., Li W., Wang H., Kuang X., Li Q., Wang Y., Xie P., Koike K. A simple and rapid method to identify and quantitatively analyze triterpenoid saponins in Ardisia crenata using ultrafast liquid chromatography coupled with electrospray ionization quadrupole mass spectrometry. J. Pharm. Biomed. Anal. 2015;102:400–408. doi: 10.1016/j.jpba.2014.10.013. [DOI] [PubMed] [Google Scholar]
- 218.Li D., Schmitz O.J. Comprehensive two-dimensional liquid chromatography tandem diode array detector (DAD) and accurate mass Q-TOF-MS for the analysis of flavonoids and iridoid glycosides in Hedyotis diffusa. Anal. Bioanal. Chem. 2015;407:231–240. doi: 10.1007/s00216-014-8057-4. [DOI] [PubMed] [Google Scholar]
- 219.Piotrowski P., Bocian S., Śliwka K., Buszewski B. Simultaneous analysis of zolpidem and its metabolite in whole blood and oral fluid samples by SPE-LC/MS for clinical and forensic purposes. Adv. Med. Sci. 2015;60:167–172. doi: 10.1016/j.advms.2015.01.010. [DOI] [PubMed] [Google Scholar]
- 220.Han L., Liu E., Kojo A., Zhao J., Li W., Zhang Y., Wang T., Gao X. Qualitative and quantitative analysis of Eclipta prostrata L. by LC/MS. Sci. World J. 2015;2015:980890. doi: 10.1155/2015/980890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.He C., Li J., Xu N., Wang R., Li Z., Yang L., Wang Z. Pharmacokinetics, bioavailability, and metabolism of Notoginsenoside Fc in rats by liquid chromatography/electrospray ionization tandem mass spectrometry. J. Pharm. Biomed. Anal. 2015;109:150–157. doi: 10.1016/j.jpba.2015.02.038. [DOI] [PubMed] [Google Scholar]
- 222.Yang T., Liu S., Zheng T.H., Tao Y.Y., Liu C.H. Comparative pharmacokinetics and tissue distribution profiles of lignin components in normal and hepatic fibrosis rats after oral administration of Fuzheng Huayu recipe. J. Ethnopharmacol. 2015;166:305–312. doi: 10.1016/j.jep.2015.03.024. [DOI] [PubMed] [Google Scholar]
- 223.Xing R., Zhou L., Xie L., Hao K., Rao T., Wang Q., Ye W., Fu H., Wang X., Wang G., et al. Development of a systematic approach to rapid classification and identification of notoginsenosides and metabolites in rat feces based on liquid chromatography coupled triple time-of-flight mass spectrometry. Anal. Chim. Acta. 2015;867:56–66. doi: 10.1016/j.aca.2015.02.039. [DOI] [PubMed] [Google Scholar]
- 224.Wang X., Liu X., Xu X., Zhu T., Shi F., Qin K., Cai B. Screening and identification of multiple constituents and their metabolites of Fangji Huangqi Tang in rats by ultra-high performance liquid chromatography coupled with quadrupole time-of-flight tandem mass spectrometry basing on coupling data processing techniques. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2015;985:14–28. doi: 10.1016/j.jchromb.2015.01.021. [DOI] [PubMed] [Google Scholar]
- 225.Su S., Duan J., Wang P., Liu P., Guo J., Shang E., Qian D., Tang Y., Tang Z. Metabolomic study of biochemical changes in the plasma and urine of primary dysmenorrhea patients using UPLC-MS coupled with a pattern recognition approach. J. Proteome Res. 2013;12:852–865. doi: 10.1021/pr300935x. [DOI] [PubMed] [Google Scholar]
- 226.Li W., Tang Y., Guo J., Shang E., Qian Y., Wang L., Zhang L., Liu P., Su S., Qian D. Comparative metabolomics analysis on hematopoietic functions of herb pair Gui-Xiong by ultra-high-performance liquid chromatography coupled to quadrupole time-of-flight mass spectrometry and pattern recognition approach. J. Chromatogr. A. 2014;1346:49–56. doi: 10.1016/j.chroma.2014.04.042. [DOI] [PubMed] [Google Scholar]
- 227.Sun L., Wei H., Zhang F. Qualitative analysis and quality control of Traditional Chinese Medicine preparation Tanreqing injection by LC-TOF/MS and HPLC-DAD-ELSD. Anal. Methods. 2013;5:6431–6440. doi: 10.1039/c3ay40681d. [DOI] [Google Scholar]
- 228.Ip S.P., Zhao M., Xian Y., Chen M., Zong Y., Tjong Y.W., Tsai S.H., Sung J.J., Bensoussan A., Berman B., et al. Quality assurance for Chinese herbal formulae: Standardization of IBS-20, a 20-herb preparation. Chin. Med. 2010;5 doi: 10.1186/1749-8546-5-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Zhang H., Wang J.R., Yau L.F., Ho H.M., Chan C.L., Hu P., Liu L., Jiang Z.H. A cellular lipidomic study on the Aβ-induced neurotoxicity and neuroprotective effects of EGCG by using UPLC/MS-based glycerolipids profiling and multivariate analysis. Mol. Biosyst. 2012;8:3208–3215. doi: 10.1039/c2mb25126d. [DOI] [PubMed] [Google Scholar]
- 230.Korecka M., Waligorska T., Figurski M. Qualification of a surrogate matrix-based absolute quantification method for amyloid-β in human cerebrospinal fluid using 2D UPLC-tandem mass spectrometry. J. Alzheimers Dis. 2014;41:441–451. doi: 10.3233/JAD-132489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Hu J., Wu Z., Yan J., Pang W., Liang D., Xu X. A promising approach for understanding the mechanism of Traditional Chinese Medicine by the aggregation morphology. J. Ethnopharmacol. 2009;123:267–274. doi: 10.1016/j.jep.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 232.Cai P.S., Zhao Y., Yang T.H., Chen J., Xiong C.M., Ruan J.L. Preparation of magnetic molecularly imprinted polymers for selective isolation and determination of kaempferol and protoapigenone in Macrothelypteris torresiana. J. Huazhong Univ. Sci. Technolog. Med. Sci. 2014;34:845–855. doi: 10.1007/s11596-014-1363-4. [DOI] [PubMed] [Google Scholar]
- 233.Luo G.A., Wang Y.M., Liang Q.L., Liu Q.F. System Biology for Traditional Chinese Medicine. John Wiley & Sons, Inc.; Hoboken, NJ, USA: 2012. [Google Scholar]
- 234.Jonker C., Hamman J.H., Kotzé A.F. Intestinal paracellular permeation enhancement with quaternised chitosan: In situ and in vitro evaluation. Int. J. Pharm. 2002;238:205–213. doi: 10.1016/S0378-5173(02)00068-6. [DOI] [PubMed] [Google Scholar]