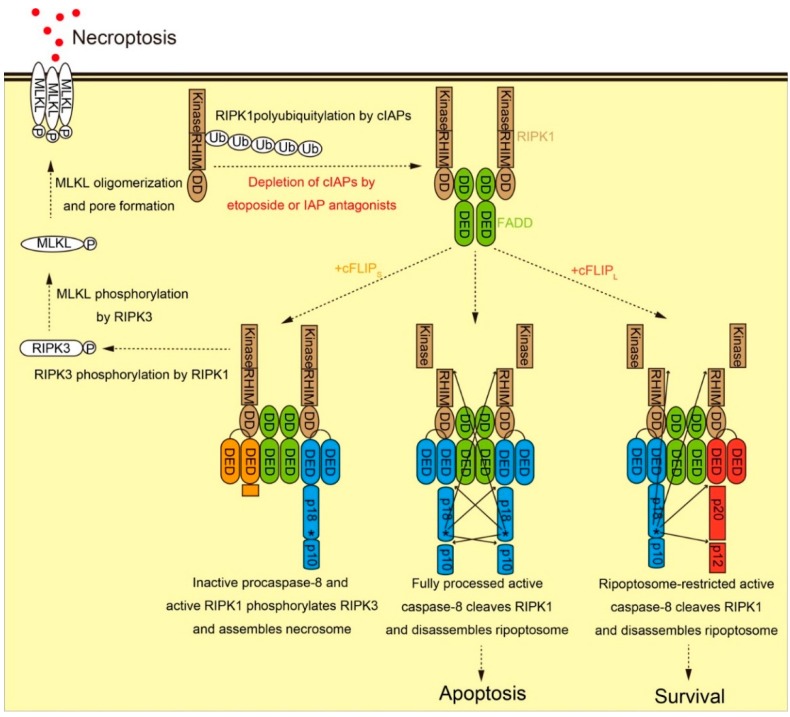
Figure 3.
Functional role of cFLIP during ripoptosome formation. RIPK1 (brown) is composed of catalytic kinase domain, RIP homotypic interaction motif (RHIM), and DD. Upon RIPK1 activation by genotoxic stress, DD of RIPK1 is exposed and binds to DD of FADD (green). Subsequently, DED-containing proteins including procaspase-8 (blue), cFLIPL (red), and cFLIPS (orange) are recruited to RIPK1-bound FADD via DED-DED interaction, thereby forming ripoptosome. Fully processed active caspase-8, generated by procaspase-8 homodimerization, activates effector caspases, cleaves RIPK1, disassembles ripoptosome, and induces apoptosis. Procaspase-8-cFLIPL heterodimer produces p43-FLIP and p22-FLIP, cleaves RIPK1, disassembles ripoptosome, but does not process procaspase-8, leading to cellular survival. In contrast, procaspase-8-cFLIPS heterodimer fails to cleave RIPK1. This leads to the assembly of necrosome composed of RIPK1-RIPK3-MLKL and the execution of necroptosis.