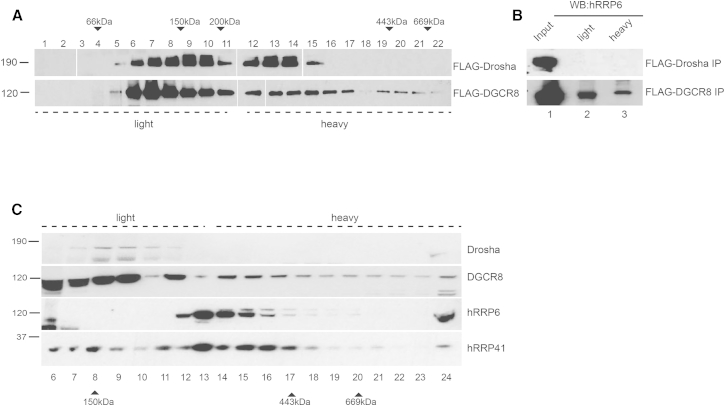
Figure 2.
DGCR8 and the Exosome Coexist in a Complex
(A) Sedimentation patterns of immunopurified FLAG-Drosha and FLAG-DGCR8 native complexes in 5%–30% glycerol gradient fractions, as revealed by western blot analysis with an anti-FLAG antibody. “Light” denotes lighter-molecular-weight fractions, whereas “heavy” indicates heavier molecular fractions. The migration of the molecular weight markers is indicated at the top (to see uncropped versions of these images, see Figure S2B).
(B) Western blot of coimmunoprecipitated hRRP6 with FLAG-Drosha (top panel) and FLAG-DGCR8 (bottom panel) after glycerol gradient fractionation. Fractions from a 5%–30% glycerol gradient were pooled into light (lane 2), corresponding to fractions 1–11, and heavy (lane 3), corresponding to fractions 12–22, and run in a single lane for sensitivity purposes.
(C) Sedimentation patterns of endogenous Drosha, DGCR8, hRRP6, and hRRP41 proteins in 5%–30% glycerol gradients from nuclear HEK293T cell extracts, as revealed by western blot analysis with specific antibodies. Lysates run in all gradients were produced in the presence of DNase and RNase.