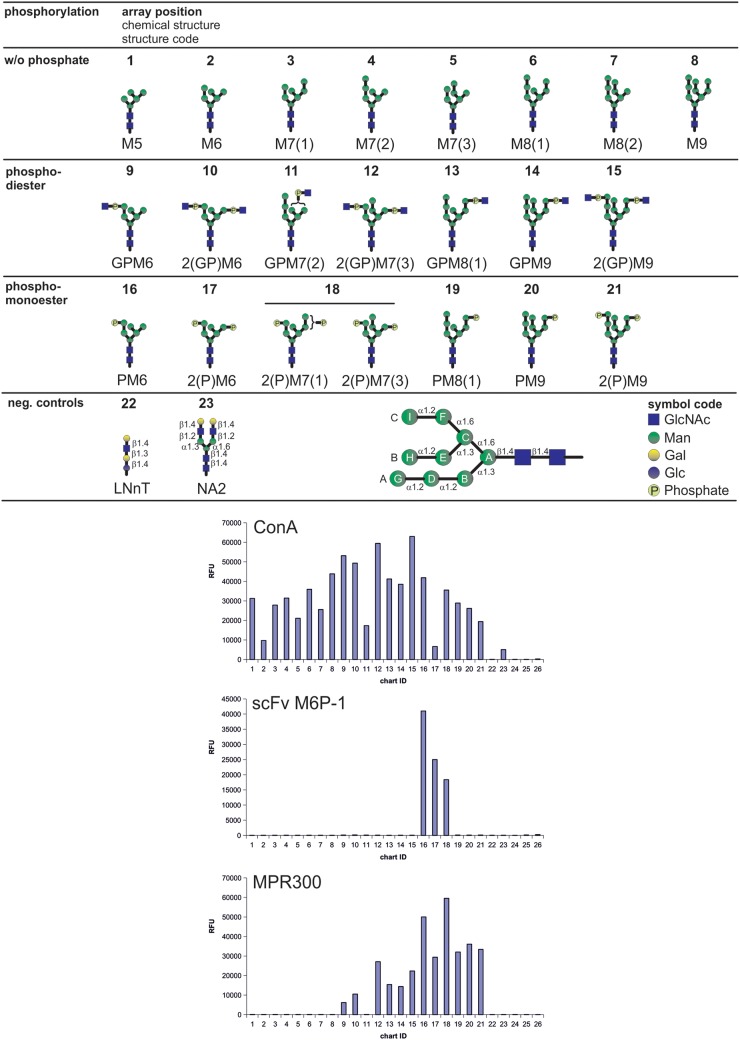
Fig. 3.
Binding of scFv M6P-1 to glycans immobilized in an array on glass slides. The glycan microarray used in this analysis contained the schematically depicted glycans that were printed in replicates of four at a concentration of 100 µM with the exception of glycans 3, 7, 11, 12, 15, 18 and 21, which were only available at lower concentrations (<100 µM). Below a structure code is given where G = GlcNAc (blue squares), P = phosphate (light yellow circles) and M = Man (green circles). Glucose and Galactose in controls are depicted as blue and yellow circles, respectively. Numbers indicate the number of residues and isomers are distinguished by numbers in brackets. N-Glycan residues are designated as schematically depicted in the lower panel following the nomenclature as published (Castonguay et al. 2012). The array was interrogated with biotinylated scFv M6P-1 and Con A and affinity purified fetal bovine serum MPR300. Bound scFv M6P-1 and Con A were quantified after incubation with Cy5-labeled streptavidin and MPR300 was assayed using a rabbit polyclonal antibody and a Cy5-labeled anti-rabbit IgG as described (Castonguay et al. 2012). Con A and MPR300 served as positive controls to validate the array. Relative fluorescence units were determined as the average of four replicates, and the glycan numbers correspond to the glycans above. Biotinylated Con A at 0.5 µg/mL (left) was detected with Cy5 Streptavidin (5 µg/mL); biotinylated scFv M6P-1 at 20 µg/mL (middle) detected with Cy5 Streptavidin (5 µg/mL); MPR300 at 5 µg/mL (right) detected with a rabbit polyclonal antibody (1:250 dilution) and Cy5-labeled goat, anti-rabbit IgG at 5 µg/mL. Slides were scanned for Cy5 in a ProScaArray scanner (PerkinElmer) and average fluorescence units were determined using the corresponding ProScanArray software.