Abstract
Obesity is an established risk factor for postmenopausal breast cancer. The mechanisms through which obesity influences the development and progression of breast cancer are not fully elucidated; however, several factors such as increased oestrogen, concentrations of various members of the insulin family and inflammation that are associated with adiposity are purported to be important factors in this relationship. Emerging research has also begun to focus on the role of adipokines, (i.e. adipocyte secreted factors), in breast cancer. Leptin secretion is directly related to adiposity and is believed to promote breast cancer directly and independently, as well as through involvement with the oestrogen and insulin signalling pathways. As leptin is secreted from white adipose tissue, any intervention that reduces adiposity may be favourable. However, it is also important to consider that energy expenditure through exercise, independent of fat loss, may improve leptin regulation. The purpose of this narrative review was to explore the role of leptin in breast cancer development and progression, identify key interactions with oestrogen and the insulin family, and distinguish the potential effects of exercise on these interactions.
Keywords: Adipokines, body composition, energy expenditure, IGF-1
Introduction
Breast cancer is the most common cancer in women worldwide (1). Obesity is an established risk factor for breast cancer occurring in postmenopausal women and contributes to 9% of all breast cancer deaths worldwide and 13% in high-income countries (2). Increased adiposity may promote the development and progression of postmenopausal breast cancer through several mechanisms, including increased circulating concentrations of oestrogen and/or insulin and insulin-like growth factor (IGF) (3) and oestrogenic persistent organic pollutants such as organochlorines (4). Emerging research demonstrates that adipokines, bioactive molecules produced and secreted by adipocytes, may also contribute to postmenopausal breast cancer.
Adipocytes produce close to 50 different adipokines that serve numerous functions in the body such as inflammation and modulating the acute phase response, insulin sensitivity, lipid metabolism, and appetite and energy balance (5). Specific to breast cancer, leptin is the most studied adipokine because of its associations with increased risk of postmenopausal breast cancer (6–11) and its known role of promoting breast cancer tumour growth and progression in vitro (12–17). This paper will focus on the roles of leptin in postmenopausal breast cancer development and progression given the plethora of evidence supporting this role. However, leptin is not the only adipokine that is purported to have implications in postmenopausal breast cancer. For example, adiponectin, whose concentrations are reduced in obesity, is hypothesized to have anti-oncogenic effects that drive the relationship between reduced adiposity and decreased breast cancer risk. This relationship is further complicated with the antagonistic actions of adiponectin against leptin (18–23). Considering the ratio between leptin and adiponectin may provide further insight into their role in oncogenesis than examining their individual absolute concentrations (24). However, it is important to understand the independent role of leptin in relation to oestrogen and the insulin family before examining the integrated role of adipokines in this discipline.
Leptin, is directly related to adiposity, with higher circulating levels in overweight/obese individuals than in lean individuals (25–27). Leptin can promote breast cancer directly and independently, as well as indirectly through the oestrogen and insulin signalling pathways (24,28,29), thereby providing the basis for hormone-mediated, pro-carcinogenic cross-talk. As leptin is secreted from white adipose tissue, any intervention that reduces adiposity may be favourable in preventing the initiation and progression of breast cancer tumourigenesis by antagonizing leptin secretion and/or leptin-mediated signalling. However, it is also important to consider that energy expenditure through exercise, independent of fat loss, may also improve leptin regulation. This narrative review will examine the potential effects of leptin in breast cancer development and progression, describe key interactions with oestrogen and the insulin family, and discuss the influence of exercise on these interactions.
Leptin
Discovery of leptin and its role in human metabolism
Leptin originates from the Greek root word ‘leptos’, meaning thin, and was discovered in 1994 through positional cloning of the ob mouse gene (30). Leptin is produced predominantly by white adipocytes, with larger-sized adipocytes producing more leptin than smaller adipocytes (31), and circulates as a 16 kDA glycosylated protein (32). Although it was originally believed that leptin was synthesized exclusively in white adipose tissue, there is evidence that it is also produced in smaller amounts in the placenta (33), gastric mucosa cells (34), myocytes (35) and mammary epithelial cells (36).
In humans, adipose tissue mass is the primary determinant of circulating leptin concentrations (37) with the ob mRNA content of adipocytes being twice as high in individuals with obesity compared with normal-weight individuals (38). Similarly, a strong correlation between total body fat and plasma leptin exists (38,39), whereby serum leptin concentrations are more than three times greater in individuals with obesity, compared with normal-weight individuals (31.3 ± 24.1 vs. 7.5 ± 9.3 ng mL−1, respectively) (38). In addition to greater production of leptin, individuals with obesity may also be insensitive to endogenous leptin, thus creating a leptin-resistant state (40,41). This leptin-resistant state is thought to be a result of several mechanisms such as defective leptin transport across the blood–brain barrier, attenuation of leptin signalling through the inactivation of the JAK-STAT pathway (via inhibition by suppressor of cytokine signalling-3), endoplasmic reticulum stress and inflammation as reviewed in (42). Given this dys-regulation in the leptin signalling cascade, greater amounts of leptin are continually released from the adipose tissue of individuals with obesity. Despite the attenuation of the JAK-STAT pathway in leptin resistance, the ability of leptin to stimulate cell proliferation is sustained through activation of other cell signalling pathways such as phosphoinositide3kinase (PI3K)/AKT pathway and mitogen-activated protein kinase pathway (MAPK) pathway (43). Therefore, leptin remains a likely candidate for promoting tumourigenesis in obesity-driven, postmenopausal breast cancer, even in a leptin-resistant state. A clear sexual dimorphism also exists whereby leptin concentrations reported in women are two- to threefold higher than men (37,38). Although it was originally believed that these differences stemmed from the disparities in the amount of adipose tissue between men and women (38), more recent studies have shown that the dimorphism persists even after adjustment for adiposity and body mass index (BMI) (39,44,45). Thus, it is possible that oestrogens may stimulate and androgens may suppress leptin production respectively (46–48).
One main function of leptin is to act as a lipostat. As leptin is secreted in proportion to fat mass and nutritional status, it signals to the hypothalamus to suppress appetite, thereby decreasing feeding and increasing thermogenesis (49). Leptin also has pleiotropic effects on neuroendocrine function, reproductive function, pubertal development, bone development, immune function, haematopoiesis, blood pressure, glucose homeostasis, fatty acid metabolism, among other physiological roles (32,50). Although many of these leptin-related processes exert systemic effects because they are regulated by receptors expressed in central tissues (i.e. hypothalamus), some processes are also peripherally mediated (i.e. leptin receptors expressed in skeletal muscle, adipose tissue, liver, etc., Reviewed in (51,52)).
Leptin receptor biology in relation to breast cancer
Leptin exerts its intracellular effects by binding to the transmembrane receptor, Ob-R (53). Consistent with the structural similarity of leptin with various cytokines, Ob-R belongs to the cytokine receptor class I superfamily (54). Alternative mRNA splicing gives rise to different isoforms of Ob-R (Ob-Ra, Ob-Rb/Ob-Rl, Ob-Rc, Ob-Rd, Ob-Re, Ob-Rf), which have similar extracellular domains and variable intracellular structures. Ob-Ra, c, d and f possess relatively short cytoplasmic domains (also known as ‘short’ isoforms), and Ob-Re is a secreted form of the leptin receptor (reviewed in (55,56)). The short isoforms have been shown to activate some signal transduction cascades, such as the MAPK (51,57,58); however, only Ob-Rb, which contains the full-length intracellular domain, has full signalling capabilities to activate the janus kinase/signal transducer and activator of transcription (JAK-STAT3) pathway, which drives the appetite suppressing effects in the hypothalamus (59).
Leptin circulates in the plasma either free or bound to leptin-binding proteins (Ob-Re) (60,61). In lean individuals, more leptin circulates in its bound form, whereas in an obese state, the majority of leptin circulates in its free form (27,62). In line with the role of class I cytokines, the main intracellular events following the binding of leptin to Ob-Rb involve receptor dimerization and activation of the JAK/STAT pathway (56), which is responsible for nuclear effects of leptin (55,56). In cancer, activation of the JAK/STAT pathway (mainly JAK2/STAT3) results in expression of genes promoting cell proliferation and resistance to apoptosis, in addition to promoting tumour angiogenesis, invasion and migration (63,64). Other pathways hypothesized to be activated by both the long and short isoforms of Ob-R include MAPK, the PI3K/phosphodiesterase 3B (PDE3B)/cyclic adenosine monophosphate pathway, and the AMPK signalling pathways (55). In cancer, these pathways have shown to promote oncogenesis (63,65–67).
Ob-Rb is expressed in various tissues including mammary tissue (56), which is thought to facilitate mammary gland development; however, its overexpression may lead to pathophysiological conditions, such as breast cancer (56,68). Therefore, leptin receptor overexpression in mammary tissue combined with the presence of increased leptin production typically observed in individuals with obesity, results in further increased risk for breast cancer. This is of particular interest for postmenopausal women, who typically carry more adipose tissue compared with premenopausal women (69–72) and consequently exhibit a body composition phenotype that favours increased circulating leptin and oestrogen concentrations. In this connection, there is a positive association between postmenopausal women with obesity and the development of breast cancer, wherein the majority of cases are oestrogen receptor (ER)-positive and exhibit a more aggressive cancer phenotype (i.e. typically larger metastatic tumours) compared with lean women (73–77). As adipose tissue acts as a primary cellular source of both oestrogen and leptin in postmenopausal women, it is important to understand the interactions between obese-adipose tissue-derived leptin and oestrogen towards the development and progression of postmenopausal breast cancer. Given that increased obesity and oestrogen are observed in patients with breast cancer (78–81), these relationships gain further significance in exploring the possible role of leptin as a growth factor in breast cancer.
Leptin and breast cancer
Deviations in leptin and Ob-R expression: implications in breast cancer
Leptin has recently been hypothesized to have a role in the development and progression of breast cancer. Evidence from epidemiological studies indicates that women with breast cancer have higher circulating concentrations of leptin vs. women in a comparable, non-malignant population (6–10). These findings are supported by a recent meta-analysis, which concluded that leptin concentrations play a role in breast cancer development and progression (11). Measuring leptin may become an important diagnostic tool, given that the mean serum leptin levels in breast cancer case groups were significantly greater than that of control groups (11). Interestingly, circulating leptin concentrations varied in different populations with healthy individuals having the lowest concentrations, followed sequentially by patients with benign breast diseases, patients with breast cancer, and the group with the greatest concentrations was patients with breast cancer with lymph node metastasis (11). Others have shown that leptin may be associated with increased risk of postmenopausal breast cancer independent of BMI (82). Increased circulating leptin concentrations also correlate positively with tumour size (12,83), and leptin and Ob-R are strongly co-expressed in breast cancer tissue (12). Taken together, these findings further strengthen the hypothesis that leptin acts as growth factor in breast cancer. Similarly, Miyoshi, et al. found that leptin and the Ob-Rb are expressed in breast cancer tissue, and patients with high serum leptin, and tissue leptin receptor expression levels were associated with a poorer prognosis (84). In addition, patients with breast cancer with coincident high tumour leptin and Ob-Rb expression exhibit a higher metastatic rate and cancer recurrence in distant organs, collectively decreasing the survival rate (11,85).
Interest in leptin as a pro-carcinogenic factor in breast cancer piqued when findings from Cleary et al. showed that ob/ob mice (leptin deficient) did not develop mammary tumours while Lep+/+ and Lep+/− mice (leptin sufficient wild type and heterozygotes) developed breast cancer in 50% and 67% of cases, respectively (86). Similarly, db/db mice (lack Ob-R) did not develop breast cancer while Ob-R+/+ and Ob-R+/−mice did develop tumours in 69% and 82% of cases, respectively (87). Numerous studies have since been performed in an attempt to elucidate the role of leptin in mammary carcinogenesis. Leptin and Ob-R (long and short isoforms) are expressed in both normal and malignant mammary tissue, although, both leptin and the Ob-R appear to be significantly overexpressed in cancer tissue relative to non-cancer epithelium (12,85,86,88,89). This finding is consistent across all breast cancer types, as Ob-R is expressed in both hormone-sensitive and hormone-independent breast cancer (13,28,59,88,90–92). Thus, leptin and the overexpression of leptin receptors in malignant tissue may be key in the development and progression of breast cancer. It is crucial to identify whether the magnitude of leptin receptor expression and/or the sensitivity of leptin signalling within the mammary tissue is enhanced to promote breast cancer. Elucidating these mechanisms may potentially influence the prognosis and outcomes of breast cancer.
Leptin signalling is related to breast cancer development and progression
In breast cancer, the pro-carcinogenic effect of leptin is hypothesized to result from an enhanced activity of leptin/Ob-R downstream signalling pathways involved in cell proliferation such as the PI3K/Akt survival pathway, the MAPK pathway, the STAT3 pathway, and up-regulation of c-myc (one target gene of STAT3), cdk2 and cyclin D1 (genes promoting cell cycle transition G1 to S) (12) (Fig. 1). This is supported by numerous in vitro studies that have shown a proliferative response in breast cancer cell lines in response to leptin (13–17). Further, leptin has been shown to increase expression of vascular endothelial growth factor (VEGF) and its receptor, VEGF receptor-1 (VEGFR-1), in breast cancer cell lines, thus promoting angiogenesis and neovascularization of tumours (13,93,94). The pro-carcinogenic effect of leptin results not only from an enhanced activity of signalling pathways involved in cell proliferation process, but also from a likely down-regulation of apoptosis in cancer cells. Leptin has been shown to up-regulate the expression of anti-apoptotic genes such as Bcl2 and survivin and to down-regulate expression of pro-apoptotic proteins such as Bax and p53 (19,59,95–97). In addition to its direct action on mammary tumour cells, leptin can stimulate cell proliferation and migration indirectly through cross-talk with oestrogen and insulin family members and the transactivation of several key pathways involved in mammary carcinogenesis (24,29,98–100).
Figure 1.
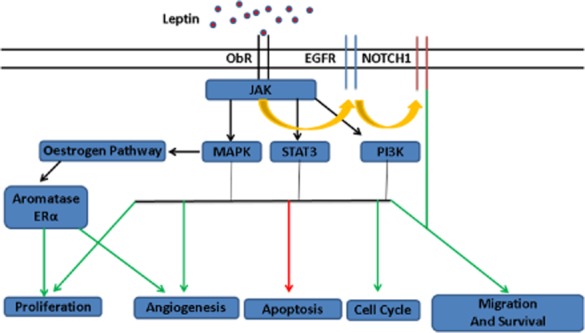
Possible pathways involved in leptin-stimulated breast cancer growth. Leptin binds to ObR on breast cancer cells, resulting in activation of multiple oncogenic pathways and various steps in tumourigenesis. Abbreviations: EGFR, epithelial growth factor receptor; MAPK, mitogen-activated protein kinase; STAT3, signal transducer and activator 3; PI3K, phosphatidylinositol 3 kinase; JAK, Janus kinase; ER-α, oestrogen receptor alpha. Green indicates stimulation, red indicates inhibition and orange arrows indicate transactivation.
Leptin and oestrogen connection
The sex steroid oestrogen is necessary for the development and function of the normal human breast. However, increased lifetime oestrogen exposure as determined by early menarche, late menopause, high bone density and obesity is associated with increased risk of postmenopausal breast cancer (101–103). Circulating levels of oestrogen are higher in women with obesity, which is associated with increased risk of developing postmenopausal breast cancer (102,104–106). Oestrogen is thought to promote mammary carcinogenesis through several mechanisms such as increased cell proliferation and cell cycle progression, inhibition of apoptosis and support of angiogenesis (73,103,107). However, emerging research indicates a functional cross-talk between oestrogen and leptin exists and may act to promote tumourigenesis.
The main source of circulating oestrogens in postmenopausal women comes from the aromatization of the C-19 steroid, androstenedione, in adipose tissue (108), a reaction catalysed by the enzyme aromatase, whose expression is increased in obesity (109). Because androstenedione production is higher in women with obesity, the resulting circulating levels of oestrogens are strongly and linearly related to adiposity (110). Plasma leptin levels are associated with total body aromatization in postmenopausal patients with breast cancer (111), and leptin has been shown to increase aromatase expression in (ER)-positive breast cancer cells (112). Leptin can also directly activate ERα (113), and increase its expression (114). Further, leptin has been shown to stabilize ERα in the presence of ICI182,780 (an anti-oestrogen drug) by interfering with the proteasome–ubiquitin pathways of ERα degradation (115) and contributes to tamoxifen resistance by inducing increased nuclear expression of ERα (116). Thus, it is not surprising that leptin is also associated with decreased effectiveness of hormone therapies (117). Further, Ob-R and ERα are co-expressed in breast cancer tissue (115,118), and Ob-R STAT-3 activation (an important pathway in leptin-induced oncogenesis) is enhanced by ERα expression (118). Lastly, oestrogen may also be able to independently stimulate leptin expression directly (98), thereby perpetuating sustained high leptin levels within the mammary tissue. For instance, in a study done by Morad et al., oestradiol concentrations in breast tissue positively correlated with mammary tissue leptin concentrations in healthy premenopausal women (98). Further, oestradiol exposure in mice bearing Mouse Mammary Tumor Virus- Polyoma Virus Middle T-Antigen (MMTV-PyMT) tumours (a murine model of breast cancer) resulted in an increase in extracellular leptin levels and expression of the leptin receptor in the mammary tumours (98). Tamoxifen treatment has also been shown to decrease leptin expression in non-malignant patients with breast cancer, further illustrating the ability of oestrogen to stimulate leptin expression in mammary tissue (98). This evidence supports the probable cross-talk between leptin and oestrogen that promotes breast cancer.
Leptin exhibits oestrogen-producing activity through multiple mechanisms including (i) increasing aromatase protein expression and enzymatic activity, thereby promoting local oestrogen production; (ii) activating ERα in the absence of its endogenous ligand (oestrogen) and (iii) improving the sensitivity of mammary tissue to oestrogens through up-regulation of ERα expression (3,59,112,113,119). As oestrogen is a known mitogen in hormone-sensitive breast cancer, it is possible that a poor breast cancer prognosis may be attributed to an interrelationship between obesity, leptin and ERα. Therefore, increases in adiposity later in adulthood and in post-menopause may increase the risk of postmenopausal, hormone-dependent breast cancer (120,121) based on the interactions between increased adiposity and elevated circulating leptin and oestrogen concentrations.
The leptin, oestrogen and insulin family triangle: interactions supporting tumour growth
As previously discussed, leptin has been shown to independently stimulate oncogenesis and promote aggressive breast cancer. However, breast cancer development and progression is multifactorial and is the result of many physiological relationships. Determining the interactions or cross-talk between leptin and other known chemopromotive growth factors (such as IGF-1 and IGF binding protein 3 [IGFBP-3] ) and hormones (such as insulin and oestrogen) in breast cancer is imperative to fully understand the mechanisms through which leptin acts in breast cancer.
Apart from its role in the regulation of blood glucose, insulin also regulates cell growth and proliferation, and can therefore exert chemopromotive effects. Obesity is associated with chronic hyperinsulinaemia (122–125) and thus, the obese phenotype may stimulate the production of IGF-1 and suppress the production of IGFBP-1 and IGFBP-3, which have been demonstrated to decrease the bioavailability and action of IGF-1 (126,127). Further, compared with normal breast tissue, breast tumours have been shown to express higher levels of the insulin receptor and exhibit a greater sensitivity to insulin (128). Therefore, despite peripheral insulin resistance associated with obesity, breast tissue may remain insulin sensitive, and in combination with obesity-associated hyperinsulinaemia (122–125) may make individuals with obesity more susceptible to insulin-mediated chemopromotive signalling within the mammary tissue. Both insulin and IGF-1 signalling promotes cell proliferation and inhibits apoptosis via downstream signalling/activation of PI3K/Akt/mTOR and Ras/Raf/MAPK pathways (109,129,130).
Leptin interacts with the IGF-1 pathway, which has pivotal implications for breast carcinogenesis, as IGF-1 and -2 are among the most potent mitogens for mammary epithelial cells (reviewed in (131)). High levels of IGF-1 and/or low levels of IGFBP-3 have generally been associated with an increased risk of breast cancer (132–134) and adverse outcomes (135,136). The IGF receptor (IGF-1R) is typically overexpressed in breast cancer and exerts several cellular actions that promote tumour growth such as supporting mitogenic processes, inhibiting apoptosis, inducing expression of VEGF, increasing cell migration and potentiating the effects of other stimulators of cell growth, including oestrogens and leptin (reviewed in (29,127,137)).
The mechanisms in which IGF-1 promotes mammary carcinogenesis in relation to leptin, however, are not consistent. Saxena and colleagues found a bidirectional cross-talk between leptin and IGF-1 signalling that transactivatesVEGFR-1 and promotes the metastatic properties, invasion and migration of breast cancer cells (29). However, another work has shown that the relationship is unidirectional with IGF-1 stimulation inducing phosphorylation and activation of Ob-R, but no effect of leptin on IGF-1 signalling (138). Further, there is accumulating evidence that IGF-1 and IGF-2 interact with the oestrogen signalling axis to regulate mitogenesis, apoptosis, adhesion, migration and differentiation of mammary epithelial cells, which could potentially have implications in breast cancer aetiology and progression (reviewed in (131)). Both insulin and IGF-1 signalling stimulate aromatase activity in adipose tissue (139), thereby promoting local oestrogen production, and in this connection IGF-1 expression is highest in ER-positive compared with ER-negative tumours (140). Further, insulin has been shown to potentiate the effects of oestrogen at the cellular level by cross-talk between oestrogen and the IGF-1 signalling pathways, both of which function either together or reciprocally as mitogens in breast cancer cells (reviewed in (141)). Collectively, these data demonstrate that cross-talk between the obesity-associated increased levels of this triad of hormones (i.e. leptin, insulin family members and oestrogen) can further perpetuate mammary tumourigenesis.
As increased adiposity is pivotal in the dys-regulation of IGF and oestrogen signalling, ultimately promoting tumourigenesis, hyperleptinaemia may further exacerbate these signals in individuals with obesity to further perpetuate the risk of breast cancer development and progression. The role of leptin in mammary tumourigenesis is multifactorial, as it acts both directly and indirectly through cross-talk with other hormones/growth factors (insulin family and oestrogen) and subsequent transactivation of other signalling pathways pertinent to breast cancer. Therefore, interventions aimed at decreasing leptin concentrations (either directly or indirectly) or abolishing its signalling capabilities may improve prognosis for patients with breast cancer.
Exercise may reduce leptin in breast cancer
Exercise has been shown to be a safe and effective adjuvant therapy for breast cancer (142). Provided that energy expenditure is in line with or exceeds energy intake, exercise can positively influence adiposity and overall body composition. Reduced adiposity has been shown to reduce circulating leptin concentrations (143,144), and this strategy could ultimately improve breast cancer prognosis. However, there is limited evidence assessing the effects of exercise on adipokine levels within both the obese and non-obese breast cancer population.
Body composition changes in breast cancer: the need for exercise
Post-diagnosis weight gain is common in patients with breast cancer, with 50–96% of women experiencing significant weight gain during and after treatment (145–147), and this is highly correlated with the type and duration of cancer treatment (147,148). Systemic treatment (adjuvant chemotherapy) is associated with greater weight gain than localized treatment (surgery and/or radiation only), and the longer the duration of treatment, the greater the weight gain (145,149–152). Many patients breast with cancer are already overweight or obese and are physically inactive at diagnosis (78), which aligns with the idea that adiposity and physical inactivity are independent risk factors for breast cancer development (153–155). Cancer diagnosis and treatment further decrease patient physical activity, with an average decline of 2 h per week from before diagnosis activity levels with a concomitant increase in sedentary activity from 1.3 h per week (pre-diagnosis) to 8.0 h per week (156). Greater decreases in physical activity from pre- to post-diagnosis were observed among breast cancer patients with obesity compared with normal-weight and overweight patients, which could potentially lead to a greater weight gain among women already with obesity (156).
Weight gain in patients with breast cancer is usually attributed to an increase in fat mass only, while lean tissues atrophy or are maintained (157,158).This altered phenotype increases the risk of developing comorbid conditions such as type 2 diabetes and cardiovascular disease, and may further impact recurrence and survival (147). Therefore, promoting physical activity during and after cancer treatment may help prevent critical changes in body composition that perpetuate altered pro-carcinogenic adipokine levels (i.e. attenuate or prevent gains in fat tissue while concomitantly maintaining or promoting gains in muscle tissue), which is critical for improving disease outcomes in patients with breast cancer.
Exercise as an adjuvant therapy in breast cancer
It is only in the last 10 years that exercise has been recognized as an adjuvant therapy following breast cancer diagnosis. Several original papers (159–162) and reviews (142,163–165) have shown that exercise is a well-tolerated and safe adjunct therapy during and after cancer treatment and results in improvements in physical functioning, quality of life and cancer-related fatigue in several cancer survivor groups. Further, a recent review concluded that increasing physical activity in breast cancer treatment and survivorship has minimal to no side effects or negative consequences at any dose, type or timing (adjuvant vs. post-adjuvant period) studied (166).
Exercise, following the diagnosis of breast cancer, has also been shown to improve survival and disease outcomes (156,166–171). A meta-analysis that included over 12,000 women reported that all levels of physical activity occurring post-diagnosis reduced breast cancer mortality by approximately 30% for women with BMI ≥ 25 kg m−2 (overweight) and decreased all-cause mortality by 41%, regardless of BMI (172). This is further supported by a recent meta-analysis that concluded that physical activity performed after diagnosis is related to a 24% reduction in mortality among breast cancer survivors (173). Taken together, this evidence supports that advocating any form of physical activity during and after breast cancer treatment will most likely provide benefits to patients with cancer and would outweigh some of the potential risks of exercise (such as increased risk for fractures and cardiovascular events with hormonal therapies, increased risk of falls associated with neuropathies related to certain types of chemotherapy, cardiac events because of treatment-related cardiotoxicity (174)); however, the underlying mechanisms that promote these benefits during exercise are unclear.
Mechanisms of exercise in breast cancer
Current hypotheses
Breast cancer aetiology is multifactorial, therefore, there has been no definitive evidence regarding the specific physiological mechanisms in which physical activity reduces breast cancer risk and improves outcomes. However, it is hypothesized that physical activity will beneficially affect adiposity, insulin resistance, and production of sex hormones, adipokines, and inflammatory markers. Here, we focus on the effects of exercise on circulating leptin, oestrogen and insulin concentrations (Fig. 2a,b).
Figure 2.
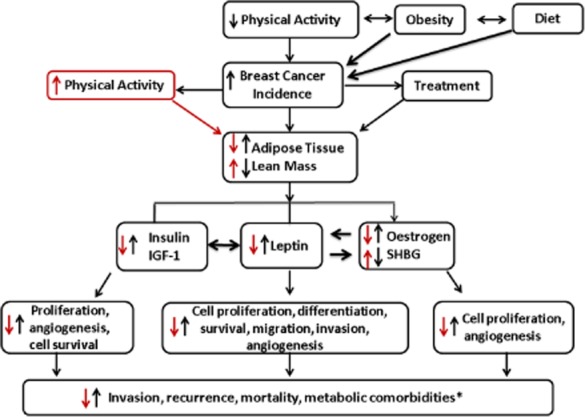
Proposed causal relationships between adiposity and outcomes in breast cancer. Potential influences obesity and physical inactivity on mammary tumourigenesis. *Increased insulin and leptin also are risk factors for other comorbidities such as type II diabetes, cardiovascular disease and metabolic syndrome. Abbreviations: IGF-1, insulin-like growth factor-1; ER-α, oestrogen receptor alpha; SHBG, sex hormone binding globulin.
Exercise improves leptin in breast cancer: adiposity vs. energy expenditure?
As leptin is under direct influence of adiposity and because it signals repletion of energy stores, any intervention that impacts fat mass, such as exercise or caloric restriction, will potentially decrease leptin concentrations. Given that patients with breast cancer are typically overweight and have a relatively high percentage of body fat at the time of diagnosis (78), they are likely to present with hyperleptinaemia at the time of diagnosis (6–10). Although leptin is hypothesized to be a growth factor in breast cancer, few studies have assessed the influence of post-diagnosis exercise on leptin concentrations in patients with breast cancer; however, the available data are promising. In a randomized control study, breast cancer survivors who lost a moderate amount of weight (median weight loss of −1.09 kg from baseline) following a 6-month, thrice weekly exercise intervention comprised of 30 min of aerobic exercise (65–85% age-predicted maximum heart rate) and 10–15 min of muscle-strengthening exercises along with individualized, hypocaloric healthy eating programme (600 kcal day−1 below estimated energy expenditure) showed a significant decrease in circulating leptin levels (175). In another study where breast cancer survivors aimed to exercise 150 min week−1, significant improvements in serum leptin concentrations were observed compared with the usual care/control group (176). Similarly, Irwin et al. showed that higher amounts of self-reported physical activity were associated with lower serum leptin concentrations in 710 women from the Health, Eating, Activity, and Lifestyle (HEAL) Study (25). If leptin is indeed associated with an increased risk of breast cancer recurrence or mortality, then its responsiveness to exercise, which is a modifiable behaviour, may be the key to improving breast cancer outcomes.
Studies investigating the effects of exercise on leptin in non-malignant populations support the use of exercise as a means to reduce leptin concentrations. The Alberta Physical Activity and Breast Cancer Prevention Trial showed that a year-long aerobic exercise intervention of 225 min week−1 significantly reduced serum leptin concentrations in previously inactive, postmenopausal women; inactivity and post-menopause are common characteristics in the breast cancer population (171). Additionally, a recent meta-analysis concluded that aerobic training significantly improved leptin levels in cancer-free, patients with type II diabetes, which are an at-risk population for breast cancer development (177–180). Numerous other studies have shown a reduction in leptin concentrations following exercise intervention (181–184), while others have not found significant reductions (185,186). The interventions in these latter studies, however, did not elicit significant reductions in adiposity, which may help to explain, at least in part, why no changes in circulating leptin levels were obtained. It is believed that only exercise interventions resulting in a reduction in fat mass will lead to decreased leptin concentrations. However, some studies have shown significant reductions in circulating levels of leptin regardless of changes in adiposity, suggesting the existence of other underlying mechanisms for this change independent of alterations in adipose tissue (187–190). It is possible that increased energy expenditure through exercise may be related to reduction in leptin. Regardless of mechanisms, promoting exercise to patients with breast cancer during and after treatment has the potential to lower leptin, and thereby possibly improving prognosis and survival.
Exercise and oestrogen: relationship with adiposity
Physical activity may influence endogenous oestrogen levels through several mechanisms including reducing body fat (191), altering serum adipokine levels (25,175,192), and reducing circulating insulin concentrations (193). The latter mechanism leads to an increase in circulating sex hormone globulin (SHBG), a protein, which binds reversibly to oestrogens to decrease their bioavailability (194). There have been several studies in postmenopausal women that support exercise as a method to decrease sex steroid concentrations and increase SHBG concentrations (192,195–197). Rock et al. demonstrated significant increases in SHBG and decreases in leptin, oestrone, oestradiol and bioavailable oestradiol in postmenopausal breast cancer survivors who attained a weight loss of at least 5% through a combined effort consisting of regular physical activity, a modest reduction in energy intake, and healthy eating attitudes and behaviours (198). This evidence is supported by recent systematic review that concluded randomized exercise trials in postmenopausal women can lower oestradiol levels on average by approximately 5–10% (199).
The causal pathway between exercise and decreased oestrogens/increased SHBG in the above studies is hypothesized to be mediated by fat loss. This relationship is plausible because adipose tissue is the main source of endogenous oestrogen in postmenopausal women (191). As oestrogen and leptin behave similarly in adapting to changes in adiposity, it is possible that leptin may be involved in mechanisms that drive these changes. Mechanisms in which exercise affects oestradiol levels independently of fat loss are not currently known. One proposed mechanism is that physical activity can cause a reduction in insulin and IGF-1 levels, which, in turn, increases SHBG levels and decreases oestradiol bioavailability (200–202). Understanding the complicated web of interactions between these hormones and leptin during exercise and fat loss may advance our understanding of the role of leptin in energy balance.
Exercise and the insulin family in breast cancer: adiposity vs. energy expenditure?
Research in numerous populations, including colorectal cancer survivors, young women with obesity, children/adolescents with obesity, as well as postmenopausal women indicates that exercise decreases fasting insulin levels (155,203–205). Few studies have examined the relationship between insulin, IGF-1 and exercise in breast cancer survivors. Higher levels of physical activity in breast cancer survivors have been associated with lower C-peptide concentrations, a marker of insulin secretion (25). Interestingly, 12 weeks of moderate-intensity Tai Chai lowered insulin concentrations, independent of fat loss, in breast cancer survivors compared with non-active patients with breast cancer (193). Another study in breast cancer survivors showed significant decreases in IGF-1, IGFBP-3, and the IGF-1 : IGFBP-3 ratio following 15 weeks of cycling without any differences in BMI or body weight between the exercise and control groups (206). The Yale Exercise and Survivorship Study, a randomized controlled trial of aerobic exercise in postmenopausal breast cancer survivors, showed moderate-intensity aerobic exercise, such as brisk walking, was associated with statistically significant decreases in IGF-1 and tended to reduce insulin concentrations (207). These studies have all shown decreases in insulin and IGF-1 following an exercise programme without a concomitant loss in body fat. Similar to leptin, it is possible that energy expenditure through exercise, rather than fat loss alone, may promote improvements in insulin and IGF-1 concentrations.
The exercise-dependent favourable change in these pro-carcinogenic factors, irrespective of fat loss, may also prove to be advantageous because the catabolic release of organochlorines that accumulate in the lipid compartments of the body would be prevented. Organochlorines is a class of persistent organic pollutants commonly found in pesticides that have oestrogenic actions (208) and have been associated with the development of oestrogen sensitive breast cancer (4). As organochlorines are lipophilic and are stored in adipose tissue, fat loss results in their release into the circulation, resulting in a potential increased risk for the development of hormone-sensitive carcinomas (209). Therefore, for this reason, fat loss in patients with breast cancer may not be as advantageous as proposed. Organochlorine concentrations/accumulation and subsequent carcinogenicity varies based on many factors including the specific organochlorine chemical class, geographical region of exposure and patient enzyme metabolism, adiposity, age, diet, etc. (210,211). Therefore, further study is required to rationalize the recommendation for maintaining or regaining adipose tissue to avoid increased circulating organochlorine levels. The potential metabolic benefits in breast cancer derived from exercise, either with or without weight loss, in addition to the improvements in quality of life and survivorship, should not be ignored because of the speculation surrounding organochlorines, adipose loss and cancer risk.
Concluding remarks
To date, the majority of the literature has focused on the relationship between adiposity and leptin, oestrogen, insulin, and breast cancer progression. Interestingly, exercise will independently decrease leptin, oestrogen and insulin concentrations. However, even more interesting is that such exercise-induced changes could occur independent of changes in fat mass. After examining the literature on exercise and its effects on this hormonal triad in breast cancer, it is becoming clear that increasing energy expenditure, irrespective of a decrease in adiposity, may have an important role in improving hormonal factors within the tumour microenvironment, which otherwise would promote mammary tumourigenesis. Therefore, future research should focus on determining the role of energy expenditure and its potential mechanisms in lowering leptin, oestrogen and insulin family member concentrations within the obese breast cancer population. Irrespective of further understanding the mechanisms by which exercise beneficially modulates this hormonal triad in breast cancer, clinicians should continue to support the use of exercise as an adjuvant therapy in breast cancer because exercise will favourably alter the net balance of most obesity-associated hormones, thereby beneficially modulating the tumour microenvironment, ultimately improving patients' quality of life and clinical outcomes.
Acknowledgments
MM was funded by the Canadian Institute for Health Research and the Ontario Ministry of Research and Innovation Early Researcher Award. SS was funded by the President's International Experience Award University of Waterloo.
Conflict of interest statement
No conflict of interest was declared.
References
- (IRAC) IAfRoC. 2012. GLOBOCAN 2012: estimated cancer incidence, mortality, and prevalence worldwide.
- 2.Danaei G, Vander Hoorn S, Lopez AD, et al. Causes of cancer in the world: comparative risk assessment of nine behavioural and environmental risk factors. Lancet. 2005;366:1784–1793. doi: 10.1016/S0140-6736(05)67725-2. [DOI] [PubMed] [Google Scholar]
- 3.Lorincz AM, Sukumar S. Molecular links between obesity and breast cancer. Endocr Relat Cancer. 2006;13:279–292. doi: 10.1677/erc.1.00729. [DOI] [PubMed] [Google Scholar]
- 4.Munoz-de-Toro M, Durando M, Beldomenico PM, et al. Estrogenic microenvironment generated by organochlorine residues in adipose mammary tissue modulates biomarker expression in ERalpha-positive breast carcinomas. Breast Cancer Res. 2006;8:R47. doi: 10.1186/bcr1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Trayhurn P, Wood IS. Adipokines: inflammation and the pleiotropic role of white adipose tissue. Br J Nutr. 2004;92:347–355. doi: 10.1079/bjn20041213. [DOI] [PubMed] [Google Scholar]
- 6.Han C, Zhang HT, Du L, et al. Serum levels of leptin, insulin, and lipids in relation to breast cancer in China. Endocrine. 2005;26:19–24. doi: 10.1385/ENDO:26:1:019. [DOI] [PubMed] [Google Scholar]
- 7.Han CZ, Du LL, Jing JX, et al. Associations among lipids, leptin, and leptin receptor gene Gin223Arg polymorphisms and breast cancer in China. Biol Trace Elem Res. 2008;126:38–48. doi: 10.1007/s12011-008-8182-z. [DOI] [PubMed] [Google Scholar]
- 8.Hancke K, Grubeck D, Hauser N, Kreienberg R, Weiss JM. Adipocyte fatty acid-binding protein as a novel prognostic factor in obese breast cancer patients. Breast Cancer Res Treat. 2010;119:367–377. doi: 10.1007/s10549-009-0577-9. [DOI] [PubMed] [Google Scholar]
- 9.Maccio A, Madeddu C, Mantovani G. Adipose tissue as target organ in the treatment of hormone-dependent breast cancer: new therapeutic perspectives. Obes Rev. 2009;10:660–670. doi: 10.1111/j.1467-789X.2009.00592.x. [DOI] [PubMed] [Google Scholar]
- 10.Ozet A, Arpaci F, Yilmaz MI, et al. Effects of tamoxifen on the serum leptin level in patients with breast cancer. Jpn J Clin Oncol. 2001;31:424–427. doi: 10.1093/jjco/hye097. [DOI] [PubMed] [Google Scholar]
- 11.Niu J, Jiang L, Guo W, Shao L, Liu Y, Wang L. The association between leptin level and breast cancer: a meta-analysis. PLoS ONE. 2013;8:e67349. doi: 10.1371/journal.pone.0067349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jarde T, Caldefie-Chezet F, Damez M, et al. Leptin and leptin receptor involvement in cancer development: a study on human primary breast carcinoma. Oncol Rep. 2008;19:905–911. [PubMed] [Google Scholar]
- 13.Zheng Q, Dunlap SM, Zhu J, et al. Leptin deficiency suppresses MMTV-Wnt-1 mammary tumor growth in obese mice and abrogates tumor initiating cell survival. Endocr Relat Cancer. 2011;18:491–503. doi: 10.1530/ERC-11-0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dieudonne MN, Machinal-Quelin F, Serazin-Leroy V, Leneveu MC, Pecquery R, Giudicelli Y. Leptin mediates a proliferative response in human MCF7 breast cancer cells. Biochem Biophys Res Commun. 2002;293:622–628. doi: 10.1016/S0006-291X(02)00205-X. [DOI] [PubMed] [Google Scholar]
- 15.Gonzalez-Perez RR, Xu Y, Guo S, Watters A, Zhou W, Leibovich SJ. Leptin upregulates VEGF in breast cancer via canonic and non-canonical signalling pathways and NFkappaB/HIF-1alpha activation. Cell Signal. 2010;22:1350–1362. doi: 10.1016/j.cellsig.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Knight BB, Oprea-Ilies GM, Nagalingam A, et al. Survivin upregulation, dependent on leptin-EGFR-Notch1 axis, is essential for leptin-induced migration of breast carcinoma cells. Endocr Relat Cancer. 2011;18:413–428. doi: 10.1530/ERC-11-0075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saxena NK, Vertino PM, Anania FA, Sharma D. Leptin-induced growth stimulation of breast cancer cells involves recruitment of histone acetyltransferases and mediator complex to CYCLIN D1 promoter via activation of Stat3. J Biol Chem. 2007;282:13316–13325. doi: 10.1074/jbc.M609798200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grossmann ME, Cleary MP. The balance between leptin and adiponectin in the control of carcinogenesis – focus on mammary tumorigenesis. Biochimie. 2012;94:2164–2171. doi: 10.1016/j.biochi.2012.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jarde T, Caldefie-Chezet F, Goncalves-Mendes N, et al. Involvement of adiponectin and leptin in breast cancer: clinical and in vitro studies. Endocr Relat Cancer. 2009;16:1197–1210. doi: 10.1677/ERC-09-0043. [DOI] [PubMed] [Google Scholar]
- 20.Ogunwobi OO, Beales IL. Globular adiponectin, acting via adiponectin receptor-1, inhibits leptin-stimulated oesophageal adenocarcinoma cell proliferation. Mol Cell Endocrinol. 2008;285:43–50. doi: 10.1016/j.mce.2008.01.023. [DOI] [PubMed] [Google Scholar]
- 21.Taliaferro-Smith L, Nagalingam A, Knight BB, Oberlick E, Saxena NK, Sharma D. Integral role of PTP1B in adiponectin-mediated inhibition of oncogenic actions of leptin in breast carcinogenesis. Neoplasia. 2013;15:23–38. doi: 10.1593/neo.121502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hou WK, Xu YX, Yu T, et al. Adipocytokines and breast cancer risk. Chin Med J. 2007;120:1592–1596. [PubMed] [Google Scholar]
- 23.Sharma D, Wang J, Fu PP, et al. Adiponectin antagonizes the oncogenic actions of leptin in hepatocellular carcinogenesis. Hepatology. 2010;52:1713–1722. doi: 10.1002/hep.23892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jarde T, Perrier S, Vasson MP, Caldefie-Chezet F. Molecular mechanisms of leptin and adiponectin in breast cancer. Eur J Cancer. 2011;47:33–43. doi: 10.1016/j.ejca.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 25.Irwin ML, McTiernan A, Bernstein L, et al. Relationship of obesity and physical activity with C-peptide, leptin, and insulin-like growth factors in breast cancer survivors. Cancer Epidemiol Biomarkers Prev. 2005;14:2881–2888. doi: 10.1158/1055-9965.EPI-05-0185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liuzzi A, Savia G, Tagliaferri M, et al. Serum leptin concentration in moderate and severe obesity: relationship with clinical, anthropometric and metabolic factors. Int J Obes Relat Metab Disord. 1999;23:1066–1073. doi: 10.1038/sj.ijo.0801036. [DOI] [PubMed] [Google Scholar]
- 27.Magni P, Liuzzi A, Ruscica M, et al. Free and bound plasma leptin in normal weight and obese men and women: relationship with body composition, resting energy expenditure, insulin-sensitivity, lipid profile and macronutrient preference. Clin Endocrinol (Oxf) 2005;62:189–196. doi: 10.1111/j.1365-2265.2005.02195.x. [DOI] [PubMed] [Google Scholar]
- 28.Rene Gonzalez R, Watters A, Xu Y, et al. Leptin-signaling inhibition results in efficient anti-tumor activity in estrogen receptor positive or negative breast cancer. Breast Cancer Res. 2009;11:R36. doi: 10.1186/bcr2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saxena NK, Taliaferro-Smith L, Knight BB, et al. Bidirectional crosstalk between leptin and insulin-like growth factor-I signaling promotes invasion and migration of breast cancer cells via transactivation of epidermal growth factor receptor. Cancer Res. 2008;68:9712–9722. doi: 10.1158/0008-5472.CAN-08-1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 31.Hamilton BS, Paglia D, Kwan AY, Deitel M. Increased obese mRNA expression in omental fat cells from massively obese humans. Nat Med. 1995;1:953–956. doi: 10.1038/nm0995-953. [DOI] [PubMed] [Google Scholar]
- 32.Ahima RS, Flier JS. Leptin. Annu Rev Physiol. 2000;62:413–437. doi: 10.1146/annurev.physiol.62.1.413. [DOI] [PubMed] [Google Scholar]
- 33.Masuzaki H, Ogawa Y, Sagawa N, et al. Nonadipose tissue production of leptin: leptin as a novel placenta-derived hormone in humans. Nat Med. 1997;3:1029–1033. doi: 10.1038/nm0997-1029. [DOI] [PubMed] [Google Scholar]
- 34.Bado A, Levasseur S, Attoub S, et al. The stomach is a source of leptin. Nature. 1998;394:790–793. doi: 10.1038/29547. [DOI] [PubMed] [Google Scholar]
- 35.Wang J, Liu R, Hawkins M, Barzilai N, Rossetti L. A nutrient-sensing pathway regulates leptin gene expression in muscle and fat. Nature. 1998;393:684–688. doi: 10.1038/31474. [DOI] [PubMed] [Google Scholar]
- 36.Smith-Kirwin SM, O'Connor DM, De Johnston J, Lancey ED, Hassink SG, Funanage VL. Leptin expression in human mammary epithelial cells and breast milk. J Clin Endocrinol Metab. 1998;83:1810–1813. doi: 10.1210/jcem.83.5.4952. [DOI] [PubMed] [Google Scholar]
- 37.Maffei M, Halaas J, Ravussin E, et al. Leptin levels in human and rodent: measurement of plasma leptin and ob RNA in obese and weight-reduced subjects. Nat Med. 1995;1:1155–1161. doi: 10.1038/nm1195-1155. [DOI] [PubMed] [Google Scholar]
- 38.Considine RV, Sinha MK, Heiman ML, et al. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med. 1996;334:292–295. doi: 10.1056/NEJM199602013340503. [DOI] [PubMed] [Google Scholar]
- 39.Thomas T, Burguera B, Melton LJ, III, et al. Relationship of serum leptin levels with body composition and sex steroid and insulin levels in men and women. Metabolism. 2000;49:1278–1284. doi: 10.1053/meta.2000.9519. [DOI] [PubMed] [Google Scholar]
- 40.Knobelspies H, Zeidler J, Hekerman P, Bamberg-Lemper S, Becker W. Mechanism of attenuation of leptin signaling under chronic ligand stimulation. BMC Biochem. 2010;11:2. doi: 10.1186/1471-2091-11-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Munzberg H, Flier JS, Bjorbaek C. Region-specific leptin resistance within the hypothalamus of diet-induced obese mice. Endocrinology. 2004;145:4880–4889. doi: 10.1210/en.2004-0726. [DOI] [PubMed] [Google Scholar]
- 42.Pan H, Guo J, Su Z. Advances in understanding the interrelations between leptin resistance and obesity. Physiol Behav. 2014;130:157–169. doi: 10.1016/j.physbeh.2014.04.003. [DOI] [PubMed] [Google Scholar]
- 43.Khan S, Shukla S, Sinha S, Meeran SM. Role of adipokines and cytokines in obesity-associated breast cancer: therapeutic targets. Cytokine Growth Factor Rev. 2013;24:503–513. doi: 10.1016/j.cytogfr.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 44.Carter S, Caron A, Richard D, Picard F. Role of leptin resistance in the development of obesity in older patients. Clin Interv Aging. 2013;8:829–844. doi: 10.2147/CIA.S36367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Saad MF, Damani S, Gingerich RL, et al. Sexual dimorphism in plasma leptin concentration. J Clin Endocrinol Metab. 1997;82:579–584. doi: 10.1210/jcem.82.2.3739. [DOI] [PubMed] [Google Scholar]
- 46.Carmina E, Ferin M, Gonzalez F, Lobo RA. Evidence that insulin and androgens may participate in the regulation of serum leptin levels in women. Fertil Steril. 1999;72:926–931. doi: 10.1016/s0015-0282(99)00387-8. [DOI] [PubMed] [Google Scholar]
- 47.Hislop MS, Ratanjee BD, Soule SG, Marais AD. Effects of anabolic-androgenic steroid use or gonadal testosterone suppression on serum leptin concentration in men. Eur J Endocrinol. 1999;141:40–46. doi: 10.1530/eje.0.1410040. [DOI] [PubMed] [Google Scholar]
- 48.Rosenbaum M, Pietrobelli A, Vasselli JR, Heymsfield SB, Leibel RL. Sexual dimorphism in circulating leptin concentrations is not accounted for by differences in adipose tissue distribution. Int J Obes Relat Metab Disord. 2001;25:1365–1371. doi: 10.1038/sj.ijo.0801730. [DOI] [PubMed] [Google Scholar]
- 49.Banks WA. The many lives of leptin. Peptides. 2004;25:331–338. doi: 10.1016/j.peptides.2004.02.014. [DOI] [PubMed] [Google Scholar]
- 50.Bjorbaek C, Kahn BB. Leptin signaling in the central nervous system and the periphery. Recent Prog Horm Res. 2004;59:305–331. doi: 10.1210/rp.59.1.305. [DOI] [PubMed] [Google Scholar]
- 51.Bjorbaek C, Uotani S, da Silva B, Flier JS. Divergent signaling capacities of the long and short isoforms of the leptin receptor. J Biol Chem. 1997;272:32686–32695. doi: 10.1074/jbc.272.51.32686. [DOI] [PubMed] [Google Scholar]
- 52.Margetic S, Gazzola C, Pegg GG, Hill RA. Leptin: a review of its peripheral actions and interactions. Int J Obes Relat Metab Disord. 2002;26:1407–1433. doi: 10.1038/sj.ijo.0802142. [DOI] [PubMed] [Google Scholar]
- 53.Tartaglia LA. The leptin receptor. J Biol Chem. 1997;272:6093–6096. doi: 10.1074/jbc.272.10.6093. [DOI] [PubMed] [Google Scholar]
- 54.Tartaglia LA, Dembski M, Weng X, et al. Identification and expression cloning of a leptin receptor, OB-R. Cell. 1995;83:1263–1271. doi: 10.1016/0092-8674(95)90151-5. [DOI] [PubMed] [Google Scholar]
- 55.Fruhbeck G. Intracellular signalling pathways activated by leptin. Biochem J. 2006;393:7–20. doi: 10.1042/BJ20051578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schulz LC, Widmaier EP. Leptin receptors. In: Castracane MC, Henson VD, editors. Lepin. New York, New York: Springer; 2007. pp. 12–31. [Google Scholar]
- 57.Hileman SM, Tornoe J, Flier JS, Bjorbaek C. Transcellular transport of leptin by the short leptin receptor isoform ObRa in Madin–Darby canine kidney cells. Endocrinology. 2000;141:1955–1961. doi: 10.1210/endo.141.6.7450. [DOI] [PubMed] [Google Scholar]
- 58.Murakami T, Yamashita T, Iida M, Kuwajima M, Shima K. A short form of leptin receptor performs signal transduction. Biochem Biophys Res Commun. 1997;231:26–29. doi: 10.1006/bbrc.1996.6030. [DOI] [PubMed] [Google Scholar]
- 59.Dubois V, Jarde T, Delort L, et al. Leptin induces a proliferative response in breast cancer cells but not in normal breast cells. Nutr Cancer. 2014;66:645–655. doi: 10.1080/01635581.2014.894104. [DOI] [PubMed] [Google Scholar]
- 60.Lammert A, Kiess W, Bottner A, Glasow A, Kratzsch J. Soluble leptin receptor represents the main leptin binding activity in human blood. Biochem Biophys Res Commun. 2001;283:982–988. doi: 10.1006/bbrc.2001.4885. [DOI] [PubMed] [Google Scholar]
- 61.Zhang J, Scarpace PJ. The soluble leptin receptor neutralizes leptin-mediated STAT3 signalling and anorexic responses in vivo. Br J Pharmacol. 2009;158:475–482. doi: 10.1111/j.1476-5381.2009.00246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Brabant G, Nave H, Mayr B, Behrend M, van Harmelen V, Arner P. Secretion of free and protein-bound leptin from subcutaneous adipose tissue of lean and obese women. J Clin Endocrinol Metab. 2002;87:3966–3970. doi: 10.1210/jcem.87.8.8758. [DOI] [PubMed] [Google Scholar]
- 63.Carpenter RL, Lo HW. STAT3 target genes relevant to human cancers. Cancers. 2014;6:897–925. doi: 10.3390/cancers6020897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Timofeeva OA, Tarasova NI, Zhang X, et al. STAT3 suppresses transcription of proapoptotic genes in cancer cells with the involvement of its N-terminal domain. Proc Natl Acad Sci U S A. 2013;110:1267–1272. doi: 10.1073/pnas.1211805110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Brown KA, Simpson ER. Obesity and breast cancer: progress to understanding the relationship. Cancer Res. 2010;70:4–7. doi: 10.1158/0008-5472.CAN-09-2257. [DOI] [PubMed] [Google Scholar]
- 66.Frankenberry KA, Skinner H, Somasundar P, McFadden DW, Vona-Davis LC. Leptin receptor expression and cell signaling in breast cancer. Int J Oncol. 2006;28:985–993. [PubMed] [Google Scholar]
- 67.Normanno N, De Luca A, Maiello MR, et al. The MEK/MAPK pathway is involved in the resistance of breast cancer cells to the EGFR tyrosine kinase inhibitor gefitinib. J Cell Physiol. 2006;207:420–427. doi: 10.1002/jcp.20588. [DOI] [PubMed] [Google Scholar]
- 68.Mantzoros CS, Magkos F, Brinkoetter M, et al. Leptin in human physiology and pathophysiology. Am J Physiol Endocrinol Metab. 2011;301:E567–E584. doi: 10.1152/ajpendo.00315.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Donato GB, Fuchs SC, Oppermann K, Bastos C, Spritzer PM. Association between menopause status and central adiposity measured at different cutoffs of waist circumference and waist-to-hip ratio. Menopause. 2006;13:280–285. doi: 10.1097/01.gme.0000177907.32634.ae. [DOI] [PubMed] [Google Scholar]
- 70.Hadji P, Bock K, Gotschalk M, et al. The influence of serum leptin concentration on bone mass assessed by quantitative ultrasonometry in pre and postmenopausal women. Maturitas. 2003;44:141–148. doi: 10.1016/s0378-5122(02)00324-9. [DOI] [PubMed] [Google Scholar]
- 71.Lee CG, Carr MC, Murdoch SJ, et al. Adipokines, inflammation, and visceral adiposity across the menopausal transition: a prospective study. J Clin Endocrinol Metab. 2009;94:1104–1110. doi: 10.1210/jc.2008-0701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sieminska L, Wojciechowska C, Foltyn W, et al. The relation of serum adiponectin and leptin levels to metabolic syndrome in women before and after the menopause. Endokrynol Pol. 2006;57:15–22. [PubMed] [Google Scholar]
- 73.Cleary MP, Grossmann ME. Minireview: obesity and breast cancer: the estrogen connection. Endocrinology. 2009;150:2537–2542. doi: 10.1210/en.2009-0070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Biglia N, Peano E, Sgandurra P, et al. Body mass index (BMI) and breast cancer: impact on tumor histopathologic features, cancer subtypes and recurrence rate in pre and postmenopausal women. Gynecol Endocrinol. 2013;29:263–267. doi: 10.3109/09513590.2012.736559. [DOI] [PubMed] [Google Scholar]
- 75.Garrisi VM, Tufaro A, Trerotoli P, et al. Body mass index and serum proteomic profile in breast cancer and healthy women: a prospective study. PLoS ONE. 2012;7:e49631. doi: 10.1371/journal.pone.0049631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kamineni A, Anderson ML, White E, et al. Body mass index, tumor characteristics, and prognosis following diagnosis of early-stage breast cancer in a mammographically screened population. Cancer Causes Control. 2013;24:305–312. doi: 10.1007/s10552-012-0115-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.van Kruijsdijk RC, van der Wall E, Visseren FL. Obesity and cancer: the role of dysfunctional adipose tissue. Cancer Epidemiol Biomarkers Prev. 2009;18:2569–2578. doi: 10.1158/1055-9965.EPI-09-0372. [DOI] [PubMed] [Google Scholar]
- 78.Bell KE, Di Sebastiano KM, Vance V, et al. A comprehensive metabolic evaluation reveals impaired glucose metabolism and dyslipidemia in breast cancer patients early in the disease trajectory. Clin Nutr. 2014;33:550–557. doi: 10.1016/j.clnu.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 79.Thomas HV, Reeves GK, Key TJ. Endogenous estrogen and postmenopausal breast cancer: a quantitative review. Cancer Causes Control. 1997;8:922–928. doi: 10.1023/a:1018476631561. [DOI] [PubMed] [Google Scholar]
- 80.Hankinson SE, Willett WC, Manson JE, et al. Plasma sex steroid hormone levels and risk of breast cancer in postmenopausal women. J Natl Cancer Inst. 1998;90:1292–1299. doi: 10.1093/jnci/90.17.1292. [DOI] [PubMed] [Google Scholar]
- 81.Sonnenschein E, Toniolo P, Terry MB, et al. Body fat distribution and obesity in pre- and postmenopausal breast cancer. Int J Epidemiol. 1999;28:1026–1031. doi: 10.1093/ije/28.6.1026. [DOI] [PubMed] [Google Scholar]
- 82.Ollberding NJ, Kim Y, Shvetsov YB, et al. Prediagnostic leptin, adiponectin, C-reactive protein, and the risk of postmenopausal breast cancer. Cancer Prev Res. 2013;6:188–195. doi: 10.1158/1940-6207.CAPR-12-0374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chen DC, Chung YF, Yeh YT, et al. Serum adiponectin and leptin levels in Taiwanese breast cancer patients. Cancer Lett. 2006;237:109–114. doi: 10.1016/j.canlet.2005.05.047. [DOI] [PubMed] [Google Scholar]
- 84.Miyoshi Y, Funahashi T, Tanaka S, et al. High expression of leptin receptor mRNA in breast cancer tissue predicts poor prognosis for patients with high, but not low, serum leptin levels. Int J Cancer. 2006;118:1414–1419. doi: 10.1002/ijc.21543. [DOI] [PubMed] [Google Scholar]
- 85.Ishikawa M, Kitayama J, Nagawa H. Enhanced expression of leptin and leptin receptor (OB-R) in human breast cancer. Clin Cancer Res. 2004;10:4325–4331. doi: 10.1158/1078-0432.CCR-03-0749. [DOI] [PubMed] [Google Scholar]
- 86.Cleary MP, Phillips FC, Getzin SC, et al. Genetically obese MMTV-TGF-alpha/Lep(ob)Lep(ob) female mice do not develop mammary tumors. Breast Cancer Res Treat. 2003;77:205–215. doi: 10.1023/a:1021891825399. [DOI] [PubMed] [Google Scholar]
- 87.Cleary MP, Juneja SC, Phillips FC, Hu X, Grande JP, Maihle NJ. Leptin receptor-deficient MMTV-TGF-alpha/Lepr(db)Lepr(db) female mice do not develop oncogene-induced mammary tumors. Exp Biol Med. 2004;229:182–193. doi: 10.1177/153537020422900207. [DOI] [PubMed] [Google Scholar]
- 88.Garofalo C, Koda M, Cascio S, et al. Increased expression of leptin and the leptin receptor as a marker of breast cancer progression: possible role of obesity-related stimuli. Clin Cancer Res. 2006;12:1447–1453. doi: 10.1158/1078-0432.CCR-05-1913. [DOI] [PubMed] [Google Scholar]
- 89.Laud K, Gourdou I, Pessemesse L, Peyrat JP, Djiane J. Identification of leptin receptors in human breast cancer: functional activity in the T47-D breast cancer cell line. Mol Cell Endocrinol. 2002;188:219–226. doi: 10.1016/s0303-7207(01)00678-5. [DOI] [PubMed] [Google Scholar]
- 90.Fiorio E, Mercanti A, Terrasi M, et al. Leptin/HER2 crosstalk in breast cancer: in vitro study and preliminary in vivo analysis. BMC Cancer. 2008;8:305. doi: 10.1186/1471-2407-8-305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Otvos L, Jr, Kovalszky I, Riolfi M, et al. Efficacy of a leptin receptor antagonist peptide in a mouse model of triple-negative breast cancer. Eur J Cancer. 2011;47:1578–1584. doi: 10.1016/j.ejca.2011.01.018. [DOI] [PubMed] [Google Scholar]
- 92.Otvos L, Jr, Surmacz E. Targeting the leptin receptor: a potential new mode of treatment for breast cancer. Expert Rev Anticancer Ther. 2011;11:1147–1150. doi: 10.1586/era.11.109. [DOI] [PubMed] [Google Scholar]
- 93.Guo S, Gonzalez-Perez RR. Notch, IL-1 and leptin crosstalk outcome (NILCO) is critical for leptin-induced proliferation, migration and VEGF/VEGFR-2 expression in breast cancer. PLoS ONE. 2011;6:e21467. doi: 10.1371/journal.pone.0021467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhou W, Guo S, Gonzalez-Perez RR. Leptin pro-angiogenic signature in breast cancer is linked to IL-1 signalling. Br J Cancer. 2011;104:128–137. doi: 10.1038/sj.bjc.6606013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Koda M, Sulkowska M, Kanczuga-Koda L, Jarzabek K, Sulkowski S. Expression of leptin and its receptor in female breast cancer in relation with selected apoptotic markers. Folia Histochem Cytobiol. 2007;45(Suppl. 1):S187–S191. [PubMed] [Google Scholar]
- 96.Nkhata KJ, Ray A, Schuster TF, Grossmann ME, Cleary MP. Effects of adiponectin and leptin co-treatment on human breast cancer cell growth. Oncol Rep. 2009;21:1611–1619. doi: 10.3892/or_00000395. [DOI] [PubMed] [Google Scholar]
- 97.Perera CN, Chin HG, Duru N, Camarillo IG. Leptin-regulated gene expression in MCF-7 breast cancer cells: mechanistic insights into leptin-regulated mammary tumor growth and progression. J Endocrinol. 2008;199:221–233. doi: 10.1677/JOE-08-0215. [DOI] [PubMed] [Google Scholar]
- 98.Morad V, Abrahamsson A, Dabrosin C. Estradiol affects extracellular leptin : adiponectin ratio in human breast tissue in vivo. J Clin Endocrinol Metab. 2014;99:3460–3467. doi: 10.1210/jc.2014-1129. [DOI] [PubMed] [Google Scholar]
- 99.Ray A. Adipokine leptin in obesity-related pathology of breast cancer. J Biosci. 2012;37:289–294. doi: 10.1007/s12038-012-9191-9. [DOI] [PubMed] [Google Scholar]
- 100.Saxena NK, Sharma D. Multifaceted leptin network: the molecular connection between obesity and breast cancer. J Mammary Gland Biol Neoplasia. 2013;18:309–320. doi: 10.1007/s10911-013-9308-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Clemons M, Goss P. Estrogen and the risk of breast cancer. N Engl J Med. 2001;344:276–285. doi: 10.1056/NEJM200101253440407. [DOI] [PubMed] [Google Scholar]
- 102.Yager JD, Davidson NE. Estrogen carcinogenesis in breast cancer. N Engl J Med. 2006;354:270–282. doi: 10.1056/NEJMra050776. [DOI] [PubMed] [Google Scholar]
- 103.Yue W, Yager JD, Wang JP, Jupe ER, Santen RJ. Estrogen receptor-dependent and independent mechanisms of breast cancer carcinogenesis. Steroids. 2013;78:161–170. doi: 10.1016/j.steroids.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 104.Baglietto L, English DR, Hopper JL, et al. Circulating steroid hormone concentrations in postmenopausal women in relation to body size and composition. Breast Cancer Res Treat. 2009;115:171–179. doi: 10.1007/s10549-008-0069-3. [DOI] [PubMed] [Google Scholar]
- 105.Key T, Appleby P, Barnes I, Reeves G, Endogenous H Breast Cancer Collaborative G. Endogenous sex hormones and breast cancer in postmenopausal women: reanalysis of nine prospective studies. J Natl Cancer Inst. 2002;94:606–616. doi: 10.1093/jnci/94.8.606. [DOI] [PubMed] [Google Scholar]
- 106.Key TJ, Appleby PN, Reeves GK, et al. Body mass index, serum sex hormones, and breast cancer risk in postmenopausal women. J Natl Cancer Inst. 2003;95:1218–1226. doi: 10.1093/jnci/djg022. [DOI] [PubMed] [Google Scholar]
- 107.Liang J, Shang Y. Estrogen and cancer. Annu Rev Physiol. 2013;75:225–240. doi: 10.1146/annurev-physiol-030212-183708. [DOI] [PubMed] [Google Scholar]
- 108.MacDonald PC, Edman CD, Hemsell DL, Porter JC, Siiteri PK. Effect of obesity on conversion of plasma androstenedione to estrone in postmenopausal women with and without endometrial cancer. Am J Obstet Gynecol. 1978;130:448–455. doi: 10.1016/0002-9378(78)90287-9. [DOI] [PubMed] [Google Scholar]
- 109.Ferguson RD, Gallagher EJ, Scheinman EJ, Damouni R, LeRoith D. The epidemiology and molecular mechanisms linking obesity, diabetes, and cancer. Vitam Horm. 2013;93:51–98. doi: 10.1016/B978-0-12-416673-8.00010-1. [DOI] [PubMed] [Google Scholar]
- 110.Rose DP, Komninou D, Stephenson GD. Obesity, adipocytokines, and insulin resistance in breast cancer. Obes Rev. 2004;5:153–165. doi: 10.1111/j.1467-789X.2004.00142.x. [DOI] [PubMed] [Google Scholar]
- 111.Geisler J, Haynes B, Ekse D, Dowsett M, Lonning PE. Total body aromatization in postmenopausal breast cancer patients is strongly correlated to plasma leptin levels. J Steroid Biochem Mol Biol. 2007;104:27–34. doi: 10.1016/j.jsbmb.2006.09.040. [DOI] [PubMed] [Google Scholar]
- 112.Catalano S, Marsico S, Giordano C, et al. Leptin enhances, via AP-1, expression of aromatase in the MCF-7 cell line. J Biol Chem. 2003;278:28668–28676. doi: 10.1074/jbc.M301695200. [DOI] [PubMed] [Google Scholar]
- 113.Catalano S, Mauro L, Marsico S, et al. Leptin induces, via ERK1/ERK2 signal, functional activation of estrogen receptor alpha in MCF-7 cells. J Biol Chem. 2004;279:19908–19915. doi: 10.1074/jbc.M313191200. [DOI] [PubMed] [Google Scholar]
- 114.Fusco R, Galgani M, Procaccini C, et al. Cellular and molecular crosstalk between leptin receptor and estrogen receptor-{alpha} in breast cancer: molecular basis for a novel therapeutic setting. Endocr Relat Cancer. 2010;17:373–382. doi: 10.1677/ERC-09-0340. [DOI] [PubMed] [Google Scholar]
- 115.Garofalo C, Sisci D, Surmacz E. Leptin interferes with the effects of the antiestrogen ICI 182,780 in MCF-7 breast cancer cells. Clin Cancer Res. 2004;10:6466–6475. doi: 10.1158/1078-0432.CCR-04-0203. [DOI] [PubMed] [Google Scholar]
- 116.Chen X, Zha X, Chen W, et al. Leptin attenuates the anti-estrogen effect of tamoxifen in breast cancer. Biomed Pharmacother. 2013;67:22–30. doi: 10.1016/j.biopha.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 117.Yom CK, Lee KM, Han W, et al. Leptin as a potential target for estrogen receptor-positive breast cancer. J Breast Cancer. 2013;16:138–145. doi: 10.4048/jbc.2013.16.2.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Binai NA, Damert A, Carra G, et al. Expression of estrogen receptor alpha increases leptin-induced STAT3 activity in breast cancer cells. Int J Cancer. 2010;127:55–66. doi: 10.1002/ijc.25010. [DOI] [PubMed] [Google Scholar]
- 119.Liu E, Samad F, Mueller BM. Local adipocytes enable estrogen-dependent breast cancer growth: role of leptin and aromatase. Adipocyte. 2013;2:165–169. doi: 10.4161/adip.23645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Boeing H. Obesity and cancer – the update 2013. Best Pract Res Clin Endocrinol Metab. 2013;27:219–227. doi: 10.1016/j.beem.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 121.Eliassen AH, Colditz GA, Rosner B, Willett WC, Hankinson SE. Adult weight change and risk of postmenopausal breast cancer. JAMA. 2006;296:193–201. doi: 10.1001/jama.296.2.193. [DOI] [PubMed] [Google Scholar]
- 122.Vague P, Juhan-Vague I, Aillaud MF, et al. Correlation between blood fibrinolytic activity, plasminogen activator inhibitor level, plasma insulin level, and relative body weight in normal and obese subjects. Metabolism. 1986;35:250–253. doi: 10.1016/0026-0495(86)90209-x. [DOI] [PubMed] [Google Scholar]
- 123.Widjaja A, Stratton IM, Horn R, Holman RR, Turner R, Brabant G. UKPDS 20: plasma leptin, obesity, and plasma insulin in type 2 diabetic subjects. J Clin Endocrinol Metab. 1997;82:654–657. doi: 10.1210/jcem.82.2.3744. [DOI] [PubMed] [Google Scholar]
- 124.Lumeng CN, Saltiel AR. Inflammatory links between obesity and metabolic disease. J Clin Invest. 2011;121:2111–2117. doi: 10.1172/JCI57132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Schelbert KB. Comorbidities of obesity. Prim Care. 2009;36:271–285. doi: 10.1016/j.pop.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 126.Nam SY, Lee EJ, Kim KR, et al. Effect of obesity on total and free insulin-like growth factor (IGF)-1, and their relationship to IGF-binding protein (BP)-1, IGFBP-2, IGFBP-3, insulin, and growth hormone. Int J Obes Relat Metab Disord. 1997;21:355–359. doi: 10.1038/sj.ijo.0800412. [DOI] [PubMed] [Google Scholar]
- 127.Renehan AG, Frystyk J, Flyvbjerg A. Obesity and cancer risk: the role of the insulin-IGF axis. Trends Endocrinol Metab. 2006;17:328–336. doi: 10.1016/j.tem.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 128.Frittitta L, Vigneri R, Papa V, Goldfine ID, Grasso G, Trischitta V. Structural and functional studies of insulin receptors in human breast cancer. Breast Cancer Res Treat. 1993;25:73–82. doi: 10.1007/BF00662403. [DOI] [PubMed] [Google Scholar]
- 129.Belardi V, Gallagher EJ, Novosyadlyy R, LeRoith D. Insulin and IGFs in obesity-related breast cancer. J Mammary Gland Biol Neoplasia. 2013;18:277–289. doi: 10.1007/s10911-013-9303-7. [DOI] [PubMed] [Google Scholar]
- 130.Pollak MN, Schernhammer ES, Hankinson SE. Insulin-like growth factors and neoplasia. Nat Rev Cancer. 2004;4:505–518. doi: 10.1038/nrc1387. [DOI] [PubMed] [Google Scholar]
- 131.Hawsawi Y, El-Gendy R, Twelves C, Speirs V, Beattie J. Insulin-like growth factor – oestradiol crosstalk and mammary gland tumourigenesis. Biochim Biophys Acta. 2013;1836:345–353. doi: 10.1016/j.bbcan.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 132.Bruning PF, Van Doorn J, Bonfrer JM, et al. Insulin-like growth-factor-binding protein 3 is decreased in early-stage operable pre-menopausal breast cancer. Int J Cancer. 1995;62:266–270. doi: 10.1002/ijc.2910620306. [DOI] [PubMed] [Google Scholar]
- 133.Key TJ, Appleby PN, Reeves GK, Roddam AW. Insulin-like growth factor 1 (IGF1), IGF binding protein 3 (IGFBP3), and breast cancer risk: pooled individual data analysis of 17 prospective studies. Lancet Oncol. 2010;11:530–542. doi: 10.1016/S1470-2045(10)70095-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Peyrat JP, Bonneterre J, Hecquet B, et al. Plasma insulin-like growth factor-1 (IGF-1) concentrations in human breast cancer. Eur J Cancer. 1993;29A:492–497. doi: 10.1016/s0959-8049(05)80137-6. [DOI] [PubMed] [Google Scholar]
- 135.Goodwin PJ, Ennis M, Pritchard KI, et al. Insulin-like growth factor binding proteins 1 and 3 and breast cancer outcomes. Breast Cancer Res Treat. 2002;74:65–76. doi: 10.1023/a:1016075709022. [DOI] [PubMed] [Google Scholar]
- 136.Law JH, Habibi G, Hu K, et al. Phosphorylated insulin-like growth factor-i/insulin receptor is present in all breast cancer subtypes and is related to poor survival. Cancer Res. 2008;68:10238–10246. doi: 10.1158/0008-5472.CAN-08-2755. [DOI] [PubMed] [Google Scholar]
- 137.Sachdev D, Yee D. The IGF system and breast cancer. Endocr Relat Cancer. 2001;8:197–209. doi: 10.1677/erc.0.0080197. [DOI] [PubMed] [Google Scholar]
- 138.Ozbay T, Nahta R. A novel unidirectional cross-talk from the insulin-like growth factor-I receptor to leptin receptor in human breast cancer cells. Mol Cancer Res. 2008;6:1052–1058. doi: 10.1158/1541-7786.MCR-07-2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Lueprasitsakul P, Latour D, Longcope C. Aromatase activity in human adipose tissue stromal cells: effect of growth factors. Steroids. 1990;55:540–544. doi: 10.1016/0039-128x(90)90049-h. [DOI] [PubMed] [Google Scholar]
- 140.Chong YM, Colston K, Jiang WG, Sharma AK, Mokbel K. The relationship between the insulin-like growth factor-1 system and the oestrogen metabolising enzymes in breast cancer tissue and its adjacent non-cancerous tissue. Breast Cancer Res Treat. 2006;99:275–288. doi: 10.1007/s10549-006-9215-y. [DOI] [PubMed] [Google Scholar]
- 141.Pichard C, Plu-Bureau G, Neves ECM, Gompel A. Insulin resistance, obesity and breast cancer risk. Maturitas. 2008;60:19–30. doi: 10.1016/j.maturitas.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 142.Jones LW, Alfano CM. Exercise-oncology research: past, present, and future. Acta Oncol (Madr) 2013;52:195–215. doi: 10.3109/0284186X.2012.742564. [DOI] [PubMed] [Google Scholar]
- 143.Halle M, Berg A, Garwers U, Grathwohl D, Knisel W, Keul J. Concurrent reductions of serum leptin and lipids during weight loss in obese men with type II diabetes. Am J Physiol. 1999;277:E277–E282. doi: 10.1152/ajpendo.1999.277.2.E277. [DOI] [PubMed] [Google Scholar]
- 144.Westerterp-Plantenga MS, Lejeune MP, Nijs I, van Ooijen M, Kovacs EM. High protein intake sustains weight maintenance after body weight loss in humans. Int J Obes Relat Metab Disord. 2004;28:57–64. doi: 10.1038/sj.ijo.0802461. [DOI] [PubMed] [Google Scholar]
- 145.Demark-Wahnefried W, Winer EP, Rimer BK. Why women gain weight with adjuvant chemotherapy for breast cancer. J Clin Oncol. 1993;11:1418–1429. doi: 10.1200/JCO.1993.11.7.1418. [DOI] [PubMed] [Google Scholar]
- 146.Rock CL, Demark-Wahnefried W. Nutrition and survival after the diagnosis of breast cancer: a review of the evidence. J Clin Oncol. 2002;20:3302–3316. doi: 10.1200/JCO.2002.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Vance V, Mourtzakis M, McCargar L, Hanning R. Weight gain in breast cancer survivors: prevalence, pattern and health consequences. Obes Rev. 2011;12:282–294. doi: 10.1111/j.1467-789X.2010.00805.x. [DOI] [PubMed] [Google Scholar]
- 148.Del Rio G, Zironi S, Valeriani L, et al. Weight gain in women with breast cancer treated with adjuvant cyclophosphomide, methotrexate and 5-fluorouracil. Analysis of resting energy expenditure and body composition. Breast Cancer Res Treat. 2002;73:267–273. doi: 10.1023/a:1015892714162. [DOI] [PubMed] [Google Scholar]
- 149.Caan BJ, Emond JA, Natarajan L, et al. Post-diagnosis weight gain and breast cancer recurrence in women with early stage breast cancer. Breast Cancer Res Treat. 2006;99:47–57. doi: 10.1007/s10549-006-9179-y. [DOI] [PubMed] [Google Scholar]
- 150.Demark-Wahnefried W, Peterson BL, Winer EP, et al. Changes in weight, body composition, and factors influencing energy balance among premenopausal breast cancer patients receiving adjuvant chemotherapy. J Clin Oncol. 2001;19:2381–2389. doi: 10.1200/JCO.2001.19.9.2381. [DOI] [PubMed] [Google Scholar]
- 151.Rock CL, Flatt SW, Newman V, et al. Factors associated with weight gain in women after diagnosis of breast cancer. Women's Healthy Eating and Living Study Group. J Am Diet Assoc. 1999;99:1212–1221. doi: 10.1016/s0002-8223(99)00298-9. [DOI] [PubMed] [Google Scholar]
- 152.Saquib N, Flatt SW, Natarajan L, et al. Weight gain and recovery of pre-cancer weight after breast cancer treatments: evidence from the Women's Healthy Eating and Living (WHEL) study. Breast Cancer Res Treat. 2007;105:177–186. doi: 10.1007/s10549-006-9442-2. [DOI] [PubMed] [Google Scholar]
- 153.Eliassen AH, Hankinson SE, Rosner B, Holmes MD, Willett WC. Physical activity and risk of breast cancer among postmenopausal women. Arch Intern Med. 2010;170:1758–1764. doi: 10.1001/archinternmed.2010.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Friedenreich CM. Review of anthropometric factors and breast cancer risk. Eur J Cancer Prev. 2001;10:15–32. doi: 10.1097/00008469-200102000-00003. [DOI] [PubMed] [Google Scholar]
- 155.Lee IM. Physical activity and cancer prevention – data from epidemiologic studies. Med Sci Sports Exerc. 2003;35:1823–1827. doi: 10.1249/01.MSS.0000093620.27893.23. [DOI] [PubMed] [Google Scholar]
- 156.Irwin ML, Crumley D, McTiernan A, et al. Physical activity levels before and after a diagnosis of breast carcinoma: the Health, Eating, Activity, and Lifestyle (HEAL) study. Cancer. 2003;97:1746–1757. doi: 10.1002/cncr.11227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Cheney CL, Mahloch J, Freeny P. Computerized tomography assessment of women with weight changes associated with adjuvant treatment for breast cancer. Am J Clin Nutr. 1997;66:141–146. doi: 10.1093/ajcn/66.1.141. [DOI] [PubMed] [Google Scholar]
- 158.Harvie MN, Campbell IT, Baildam A, Howell A. Energy balance in early breast cancer patients receiving adjuvant chemotherapy. Breast Cancer Res Treat. 2004;83:201–210. doi: 10.1023/B:BREA.0000014037.48744.fa. [DOI] [PubMed] [Google Scholar]
- 159.Backman M, Wengstrom Y, Johansson B, et al. A randomized pilot study with daily walking during adjuvant chemotherapy for patients with breast and colorectal cancer. Acta Oncol (Madr) 2014;53:510–520. doi: 10.3109/0284186X.2013.873820. [DOI] [PubMed] [Google Scholar]
- 160.Bower JE, Greendale G, Crosswell AD, et al. Yoga reduces inflammatory signaling in fatigued breast cancer survivors: a randomized controlled trial. Psychoneuroendocrinology. 2014;43:20–29. doi: 10.1016/j.psyneuen.2014.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Casla S, Hojman P, Cubedo R, Calvo I, Sampedro J, Barakat R. Integrative exercise and lifestyle intervention increases leisure-time activity in breast cancer patients. Integr Cancer Ther. 2014;13:493–501. doi: 10.1177/1534735414541962. [DOI] [PubMed] [Google Scholar]
- 162.Travier N, Fonseca-Nunes A, Javierre C, et al. Effect of a diet and physical activity intervention on body weight and nutritional patterns in overweight and obese breast cancer survivors. Med Oncol. 2014;31:783. doi: 10.1007/s12032-013-0783-5. [DOI] [PubMed] [Google Scholar]
- 163.McNeely ML, Campbell KL, Rowe BH, Klassen TP, Mackey JR, Courneya KS. Effects of exercise on breast cancer patients and survivors: a systematic review and meta-analysis. CMAJ. 2006;175:34–41. doi: 10.1503/cmaj.051073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Mishra SI, Scherer RW, Geigle PM, et al. Exercise interventions on health-related quality of life for cancer survivors. Cochrane Database Syst Rev. 2012;(8) doi: 10.1002/14651858.CD007566.pub2. CD007566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Sturgeon KM, Ky B, Libonati JR, Schmitz KH. The effects of exercise on cardiovascular outcomes before, during, and after treatment for breast cancer. Breast Cancer Res Treat. 2014;143:219–226. doi: 10.1007/s10549-013-2808-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Brenner DR, Neildon HK, Courneya KS, Friedenreich CM. Physical activity after breast cancer: effect on survival and patient-reported outcomes. Curr Breast Cancer Rep. 2014;6:193–204. [Google Scholar]
- 167.Ballard-Barbash R, Friedenreich CM, Courneya KS, Siddiqi SM, McTiernan A, Alfano CM. Physical activity, biomarkers, and disease outcomes in cancer survivors: a systematic review. J Natl Cancer Inst. 2012;104:815–840. doi: 10.1093/jnci/djs207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Bertram LA, Stefanick ML, Saquib N, et al. Physical activity, additional breast cancer events, and mortality among early-stage breast cancer survivors: findings from the WHEL Study. Cancer Causes Control. 2011;22:427–435. doi: 10.1007/s10552-010-9714-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Chen X, Lu W, Zheng W, et al. Exercise after diagnosis of breast cancer in association with survival. Cancer Prev Res. 2011;4:1409–1418. doi: 10.1158/1940-6207.CAPR-10-0355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Davies NJ, Batehup L, Thomas R. The role of diet and physical activity in breast, colorectal, and prostate cancer survivorship: a review of the literature. Br J Cancer. 2011;105(Suppl. 1):S52–S73. doi: 10.1038/bjc.2011.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Friedenreich CM. Physical activity and breast cancer: review of the epidemiologic evidence and biologic mechanisms. Recent Results Cancer Res. 2011;188:125–139. doi: 10.1007/978-3-642-10858-7_11. [DOI] [PubMed] [Google Scholar]
- 172.Ibrahim EM, Al-Homaidh A. Physical activity and survival after breast cancer diagnosis: meta-analysis of published studies. Med Oncol. 2011;28:753–765. doi: 10.1007/s12032-010-9536-x. [DOI] [PubMed] [Google Scholar]
- 173.Schmid D, Leitzmann MF. Association between physical activity and mortality among breast cancer and colorectal cancer survivors: a systematic review and meta-analysis. Ann Oncol. 2014;25:1293–1311. doi: 10.1093/annonc/mdu012. [DOI] [PubMed] [Google Scholar]
- 174.Schmitz KH, Courneya KS, Matthews C, et al. American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Med Sci Sports Exerc. 2010;42:1409–1426. doi: 10.1249/MSS.0b013e3181e0c112. [DOI] [PubMed] [Google Scholar]
- 175.Scott E, Daley AJ, Doll H, et al. Effects of an exercise and hypocaloric healthy eating program on biomarkers associated with long-term prognosis after early-stage breast cancer: a randomized controlled trial. Cancer Causes Control. 2013;24:181–191. doi: 10.1007/s10552-012-0104-x. [DOI] [PubMed] [Google Scholar]
- 176.Rogers LQ, Fogleman A, Trammell R, et al. Effects of a physical activity behavior change intervention on inflammation and related health outcomes in breast cancer survivors: pilot randomized trial. Integr Cancer Ther. 2013;12:323–335. doi: 10.1177/1534735412449687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.De Bruijn KM, Arends LR, Hansen BE, Leeflang S, Ruiter R, van Eijck CH. Systematic review and meta-analysis of the association between diabetes mellitus and incidence and mortality in breast and colorectal cancer. Br J Surg. 2013;100:1421–1429. doi: 10.1002/bjs.9229. [DOI] [PubMed] [Google Scholar]
- 178.Hayashino Y, Jackson JL, Hirata T, et al. Effects of exercise on C-reactive protein, inflammatory cytokine and adipokine in patients with type 2 diabetes: a meta-analysis of randomized controlled trials. Metabolism. 2014;63:431–440. doi: 10.1016/j.metabol.2013.08.018. [DOI] [PubMed] [Google Scholar]
- 179.Larsson SC, Mantzoros CS, Wolk A. Diabetes mellitus and risk of breast cancer: a meta-analysis. Int J Cancer. 2007;121:856–862. doi: 10.1002/ijc.22717. [DOI] [PubMed] [Google Scholar]
- 180.Liao S, Li J, Wei W, et al. Association between diabetes mellitus and breast cancer risk: a meta-analysis of the literature. Asian Pac J Cancer Prev. 2011;12:1061–1065. [PubMed] [Google Scholar]
- 181.Bouassida A, Chamari K, Zaouali M, Feki Y, Zbidi A, Tabka Z. Review on leptin and adiponectin responses and adaptations to acute and chronic exercise. Br J Sports Med. 2010;44:620–630. doi: 10.1136/bjsm.2008.046151. [DOI] [PubMed] [Google Scholar]
- 182.Damaso AR, da Silveira Campos RM, Caranti DA, et al. Aerobic plus resistance training was more effective in improving the visceral adiposity, metabolic profile and inflammatory markers than aerobic training in obese adolescents. J Sports Sci. 2014;32:1435–1445. doi: 10.1080/02640414.2014.900692. [DOI] [PubMed] [Google Scholar]
- 183.Ishii T, Yamakita T, Yamagami K, et al. Effect of exercise training on serum leptin levels in type 2 diabetic patients. Metabolism. 2001;50:1136–1140. doi: 10.1053/meta.2001.26745. [DOI] [PubMed] [Google Scholar]
- 184.Kurose S, Tsutsumi H, Yamanaka Y, et al. Improvement in endothelial function by lifestyle modification focused on exercise training is associated with insulin resistance in obese patients. Obes Res Clin Pract. 2014;8:e106–e114. doi: 10.1016/j.orcp.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 185.Ahmadizad S, Haghighi AH, Hamedinia MR. Effects of resistance vs. endurance training on serum adiponectin and insulin resistance index. Eur J Endocrinol. 2007;157:625–631. doi: 10.1530/EJE-07-0223. [DOI] [PubMed] [Google Scholar]
- 186.Llanos AA, Krok JL, Peng J, et al. Effects of a walking intervention using mobile technology and interactive voice response on serum adipokines among postmenopausal women at increased breast cancer risk. Horm Cancer. 2014;5:98–103. doi: 10.1007/s12672-013-0168-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Botero JP, Shiguemoto GE, Prestes J, et al. Effects of long-term periodized resistance training on body composition, leptin, resistin and muscle strength in elderly post-menopausal women. J Sports Med Phys Fitness. 2013;53:289–294. [PubMed] [Google Scholar]
- 188.Hickey MS, Houmard JA, Considine RV, et al. Gender-dependent effects of exercise training on serum leptin levels in humans. Am J Physiol. 1997;272:E562–E566. doi: 10.1152/ajpendo.1997.272.4.E562. [DOI] [PubMed] [Google Scholar]
- 189.Ordonez FJ, Fornieles-Gonzalez G, Camacho A, et al. Anti-inflammatory effect of exercise, via reduced leptin levels, in obese women with Down syndrome. Int J Sport Nutr Exerc Metab. 2013;23:239–244. [PubMed] [Google Scholar]
- 190.Reseland JE, Anderssen SA, Solvoll K, et al. Effect of long-term changes in diet and exercise on plasma leptin concentrations. Am J Clin Nutr. 2001;73:240–245. doi: 10.1093/ajcn/73.2.240. [DOI] [PubMed] [Google Scholar]
- 191.Longcope C, Bourget C, Flood C. The production and aromatization of dehydroepiandrosterone in post-menopausal women. Maturitas. 1982;4:325–332. doi: 10.1016/0378-5122(82)90065-2. [DOI] [PubMed] [Google Scholar]
- 192.Friedenreich CM, Woolcott CG, McTiernan A, et al. Alberta physical activity and breast cancer prevention trial: sex hormone changes in a year-long exercise intervention among postmenopausal women. J Clin Oncol. 2010;28:1458–1466. doi: 10.1200/JCO.2009.24.9557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Janelsins MC, Davis PG, Wideman L, et al. Effects of Tai Chi Chuan on insulin and cytokine levels in a randomized controlled pilot study on breast cancer survivors. Clin Breast Cancer. 2011;11:161–170. doi: 10.1016/j.clbc.2011.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Anderson DC. Sex-hormone-binding globulin. Clin Endocrinol (Oxf) 1974;3:69–96. doi: 10.1111/j.1365-2265.1974.tb03298.x. [DOI] [PubMed] [Google Scholar]
- 195.Campbell KL, Foster-Schubert KE, Alfano CM, et al. Reduced-calorie dietary weight loss, exercise, and sex hormones in postmenopausal women: randomized controlled trial. J Clin Oncol. 2012;30:2314–2326. doi: 10.1200/JCO.2011.37.9792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.McTiernan A, Tworoger SS, Ulrich CM, et al. Effect of exercise on serum estrogens in postmenopausal women: a 12-month randomized clinical trial. Cancer Res. 2004;64:2923–2928. doi: 10.1158/0008-5472.can-03-3393. [DOI] [PubMed] [Google Scholar]
- 197.Kim JW, Kim DY. Effects of aerobic exercise training on serum sex hormone binding globulin, body fat index, and metabolic syndrome factors in obese postmenopausal women. Metab Syndr Relat Disord. 2012;10:452–457. doi: 10.1089/met.2012.0036. [DOI] [PubMed] [Google Scholar]
- 198.Rock CL, Pande C, Flatt SW, et al. Favorable changes in serum estrogens and other biologic factors after weight loss in breast cancer survivors who are overweight or obese. Clin Breast Cancer. 2013;13:188–195. doi: 10.1016/j.clbc.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Neilson HK, Conroy SM, Friedenreich CM. The influence of energetic factors on biomarkers of postmenopausal breast cancer risk. Curr Nutr Rep. 2014;3:22–34. doi: 10.1007/s13668-013-0069-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Kaaks R. Nutrition, hormones, and breast cancer: is insulin the missing link? Cancer Causes Control. 1996;7:605–625. doi: 10.1007/BF00051703. [DOI] [PubMed] [Google Scholar]
- 201.Pugeat M, Crave JC, Elmidani M, et al. Pathophysiology of sex hormone binding globulin (SHBG): relation to insulin. J Steroid Biochem Mol Biol. 1991;40:841–849. doi: 10.1016/0960-0760(91)90310-2. [DOI] [PubMed] [Google Scholar]
- 202.Kaaks R, Lukanova A. Energy balance and cancer: the role of insulin and insulin-like growth factor-I. Proc Nutr Soc. 2001;60:91–106. doi: 10.1079/pns200070. [DOI] [PubMed] [Google Scholar]
- 203.Garcia-Hermoso A, Saavedra JM, Escalante Y, Sanchez-Lopez M, Martinez-Vizcaino V. Endocrinology and adolescence: aerobic exercise reduces insulin resistance markers in obese youth: a meta-analysis of randomized controlled trials. Eur J Endocrinol. 2014;171:R163–R171. doi: 10.1530/EJE-14-0291. [DOI] [PubMed] [Google Scholar]
- 204.Trapp EG, Chisholm DJ, Freund J, Boutcher SH. The effects of high-intensity intermittent exercise training on fat loss and fasting insulin levels of young women. Int J Obes. 2008;32:684–691. doi: 10.1038/sj.ijo.0803781. [DOI] [PubMed] [Google Scholar]
- 205.Wieczorek-Baranowska A, Nowak A, Michalak E, et al. Effect of aerobic exercise on insulin, insulin-like growth factor-1 and insulin-like growth factor binding protein-3 in overweight and obese postmenopausal women. J Sports Med Phys Fitness. 2011;51:525–532. [PubMed] [Google Scholar]
- 206.Fairey AS, Courneya KS, Field CJ, Bell GJ, Jones LW, Mackey JR. Effects of exercise training on fasting insulin, insulin resistance, insulin-like growth factors, and insulin-like growth factor binding proteins in postmenopausal breast cancer survivors: a randomized controlled trial. Cancer Epidemiol Biomarkers Prev. 2003;12:721–727. [PubMed] [Google Scholar]
- 207.Irwin ML, Varma K, Alvarez-Reeves M, et al. Randomized controlled trial of aerobic exercise on insulin and insulin-like growth factors in breast cancer survivors: the Yale Exercise and Survivorship study. Cancer Epidemiol Biomarkers Prev. 2009;18:306–313. doi: 10.1158/1055-9965.EPI-08-0531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Bustos S, Denegri JC, Diaz F, Tchernitchin AN. p,p′-DDT is an estrogenic compound. Bull Environ Contam Toxicol. 1988;41:496–501. doi: 10.1007/BF02020992. [DOI] [PubMed] [Google Scholar]
- 209.Pelletier C, Imbeault P, Tremblay A. Energy balance and pollution by organochlorines and polychlorinated biphenyls. Obes Rev. 2003;4:17–24. doi: 10.1046/j.1467-789x.2003.00085.x. [DOI] [PubMed] [Google Scholar]
- 210.Khanjani N, Hoving JL, Forbes AB, Sim MR. Systematic review and meta-analysis of cyclodiene insecticides and breast cancer. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2007;25:23–52. doi: 10.1080/10590500701201711. [DOI] [PubMed] [Google Scholar]
- 211.Rusiecki JA, Matthews A, Sturgeon S, et al. A correlation study of organochlorine levels in serum, breast adipose tissue, and gluteal adipose tissue among breast cancer cases in India. Cancer Epidemiol Biomarkers Prev. 2005;14:1113–1124. doi: 10.1158/1055-9965.EPI-04-0356. [DOI] [PubMed] [Google Scholar]


