STRUCTURED ABSTRACT
Objective
The goal of this study is to quantify the number of medications administered to burn patients and identify potential drugs interfering with laboratory testing.
Methods
We reviewed the medical records of 12 adult (age ≥ 18 years) burn patients with ≥ 20% total body surface area (TBSA) burns from an existing glucose control database at our institution. Dose, interval, and route of medications administered from admission to discontinuation of intensive insulin therapy were recorded. Interfering drugs were identified based on established clinical chemistry literature.
Results
The retrospective cohort of adult burn patients exhibited a mean (SD) age of 37.9 (3.0) years. Mean TBSA burn was 51.3 (9.3) %. Disease severity determined by the average multiple organ dysfunction score was 5.4 (0.2). Mean and median medications administered per day were 42.1 (9.5) and 49 (with a daily range of 0 to 65) respectively. A total of 666 potential laboratory test interferences caused by medications were identified. There were 261 different effects (e.g., increased glucose, decreased potassium). Multiple interferences, 71.0% (475/666), were caused by more than one medication.
Conclusions
Investigation of the number of medications administered to a burn patient and delineation of potential laboratory test interferences has not been conducted in burn patients. Given the substantial number of medications administered to burn patients, physicians and laboratory personnel should work together to identify potential interferences and define appropriate countermeasures while enhancing the laboratorians understanding of this unique population. This synergistic partnership can lead to intelligent support tools and potentially autocorrecting instruments.
Keywords: Medication, Burn, Interference, Clinical, Laboratory, Testing
INTRODUCTION
Burn patients represent a high-risk critically ill population. Treatment of severe burns is a multifaceted process where burn physicians must manage not only the burn injury, but also determine the appropriate volume of fluid resuscitation, assess organ dysfunction severity and functionality, calculate nutrition requirements, and monitor for signs of potential infections. [1] Medications are instrumental in treating the litany of medical complications found in burn patients and routine laboratory testing provides important objective means to do so. Unfortunately many of these medications can interfere with laboratory testing by altering the correct results, thus impairing clinical decision-making. [2]
Intensive pharmacotherapy is common in burn care. An example of burn specific pharmacotherapy interfering with laboratory testing has been recently recognized during high dose ascorbic acid therapy. [3,4] During acute burn shock patients are resuscitated using the Parkland formula. [5,6] Patients who do not respond to standard resuscitation protocols are at risk of volume overload, which has been shown to lead to extremity or abdominal compartment syndrome as well as acute respiratory distress syndrome. [7–9] Pharmacotherapy using high dose ascorbic acid (i.e., vitamin C) has been proposed to reduce fluid requirements during burn shock. [10,11] Ascorbic acid is a strong antioxidant and has been known to interfere with electrochemical reactions in a variety of laboratory tests including those for glucose, urinalysis, and creatinine. [3,12]
The myriad administered medications necessitate routine monitoring of drug-to-drug interactions by hospital pharmacists. [13] While this prevents adverse reactions within the patient, it does nothing for clinical laboratory testing. As seen with high dose ascorbic acid therapy, medications may have unintended effects on laboratory tests. These effects are well documented within the clinical laboratory community. [12,14] To our knowledge, medications administered in burn patients during high-risk time periods correlated with the number of potential laboratory testing interferences (Table 1) present is not a well-studied interaction. The goal of this study is to quantify the number of medications administered to burn patients during this phase, identify potential drug interferences that may impact routine laboratory results, and provide recommendations to improve the safety of laboratory testing in burn patients.
Table 1.
Laboratory Interferences Caused by Administered Medications
| Drug | Sample Type | Interference | Laboratory Effect |
|---|---|---|---|
| Acetaminophen | Serum | Uric Acid ↑ | Falsely High Values With Phosphotungstate Methods |
| Urine | Uric Acid ↑ | Falsely High Values With Phosphotungstate Methods | |
| Acetylsalicylic Acid | Cerebrospinal Fluid | Protein ↑ | False + with Folin-Ciocalteu Reagent |
| Serum | Albumin ↓ | Decreased Dye Binding Capacity | |
| Serum | Barbiturate ↑ | May Interfere with UV Spectrophotometry | |
| Serum | Calcium Bilirubin ↓ | Depresses Fluorescence of Calcein Method | |
| Serum | Cholesterol ↑ | Alleged Effect | |
| Serum | Uric Acid ↑ | Acts as Reducing Substance with Non-Specific Methods | |
| Urine | Acetoacetate ↑ | Reacts with Gerhardt Ferric Chloride Procedure | |
| Urine | Catecholamines ↑ | Interfering Fluorescence in Many Procedures | |
| Urine | Dihydroxyphenylalanine Screen + | Light Amber Color Produced | |
| Urine | Fouchet Test + | Produces Purple Color | |
| Urine | Glucose ↓ | Glucose Oxidase Methods Inhibited by Gentisic Acid | |
| Urine | Homogentisic Acid ↑ | Interferes with Measurement Procedure | |
| Urine | Ketones ↑ | Reddish Color with Gerhardt’s Test | |
| Urine | Phenylketones + | Purple with Ferric Chloride, Purple with Phenistix | |
| Urine | Protein ↑ | Interference with Folin-Ciocalteu Reaction | |
| Urine | Sugar ↑ | False + with Clinitest or Benedict’s | |
| Urine | Uric Acid ↑ | Acts as Reducing Substance with Non-Specific Methods | |
| Urine | UA Sugar ↑ | Conjugate May React with Benedict’s | |
| Urine | Vanillylmandelic Acid ↑ | Interferes with Fluoro-, Colorimetric Procedures | |
| Urine | 17 Hydroxy Corticosteroids ↓ | Conjugate Inhibits B-Glucuronidase, Dose > 4.8g/day | |
| Albumin | Cerebrospinal Fluid | Protein ↓ | Turbidity < Globulins With Sulfosalicylic Acid |
| Serum | Thymol Turbidity ↑ | If High | |
| Ascorbic Acid | Fecal | Occult Blood Negative | Interferes with Analytic Methods |
| Plasma | Catecholamines ↑ | Concentrated Solutions Cause Striking Fluorescence | |
| Serum | Bilirubin ↑ | At Therapeutic Concentration May Affect Sequential Multiple Analyzer 12/60 Method | |
| Serum | Creatinine ↑ | Chromogenicity in Color Reaction (Acts as Reducing Agent) | |
| Serum | Glucose ↓ | Slight Effect With Coupled Glucose Oxidase Method | |
| Serum | Glucose ↑ | At 1 mmol/L Affects Sequential Multiple Analyzer 12/60 Method | |
| Serum | Glucose ↑ | Affects Alkaline Perricyanide Methods | |
| Serum | Lactic Dehydrogenase ↓ | At Therapeutic Concentration May Depress Sequential Multiple Analyzer 12/60 Value | |
| Serum | SGOT↑ | At 1 mmol/L Affects Sequential Multiple Analyzer 12/60 Method | |
| Serum | Uric Acid ↑ | Measured as Reducing Substance | |
| ODTC | Protein ↑ | Reacts With Folin-Ciocalteu of Lowry Procedure | |
| Urine | Creatine ↑ | Acts as Reducing Agent | |
| Urine | Glucose ↓ | Impaired Color Development of Chromogen in Glucose Oxidase Method | |
| Urine | Porphobilinogen ↓ | Inhibition of Color Develop if No Prior Separation | |
| Urine | Sugar ↑ | False + With Benedict’s and Clinitest | |
| Urine | Uric Acid ↑ | Measured as Reducing Substance | |
| Urine | UA Glucose ↓ | May Inhibit Testapea and Clinistix | |
| Urine | UA Hemoglobin ↓ | In Large Amounts Inhibits Guaic Test | |
| Urine | 17 Hydroxy Corticosteroids ↑ | Interferes With Method of Reddy | |
| Calcium Gluconate | Serum | Magnesium ↓ | False ↓ if Measured by Titan-Yellow |
| Urine | Magnesium ↓ | False ↓ if Measured by Titan-Yellow | |
| Urine | 17 Hydroxy Corticosteroids ↓ | Reduced Value Reported in a Single Case | |
| Chloral Hydrate | Serum | Urea Nitrogen ↑ | Reacts with Nessler Reaction |
| Urine | Catecholamines ↑ | Interferes with Fluorometric Procedures | |
| Urine | UA Sugar ↑ | Excreted as Glucuronide, Reduces Benedict’s | |
| Urine | 17 Hydroxy Corticosteroids ↑ | Interferes with Porter-Silber Reaction | |
| Chlorpromazine | Cerebrospinal Fluid | Protein ↑ | Reacts as if Phenol with Folin-Ciocalteu Reagent |
| Serum | Glucose ↑ | Abnormally High with Repeated Doses | |
| Serum | Vitamin B12 ↓ | Possible Inhibition Effect on Some Strains of E. Gracilis | |
| ODTC | Urea Nitrogen ↑ | Produces Turbidity with Berthelot’s Reagent | |
| Urine | Metanephrines Total ↑ | Interference in Pisano Procedure | |
| Urine | Phenylketones + | Light Purple with Ferric Chloride, Same with Phenistix | |
| Urine | Porphobilinogen ↑ | Reacts with Ehrlich’s Aldehyde Reagent | |
| Urine | Pregnancy Tests + | Gives False + with Frog, Rabbit and Immunology Test | |
| Urine | UA Bile ↑ | Alleged Interference with Bili-Labstix | |
| Urine | UA Protein ↑ | Affects Turbidity Tests For Up to 3 Days | |
| Urine | Urobilinogen ↑ | Reacts with Ehrlich’s Aldehyde Reagent | |
| Urine | 17 Ketogenic Steroids ↑ | Interferes with Zimmerman Reaction | |
| Urine | 17 Ketosteroids ↑ | Interferes with Zimmerman Reaction | |
| Urine | 17 Hydroxy Corticosteroids ↑ | Interferes with Porter-Silber Reaction | |
| Urine | 5 Hydroxy Indoleacetic Acid ↓ | Interferes with Method of Goldenberg | |
| Copper | Serum | Acid Phosphatase Total ↓ | Cupric Ions Inhibit Red Cell Enzyme |
| Serum | Calcium ↑ | Interferes with Ethylenediaminetetraacetic Acid Titration Procedures | |
| Serum | Protein-Bound Iodine ↓ | As Contaminant of Water May Affect Analysis | |
| Serum | Sodium ↑ | May Interfere with Flame Photometry | |
| Urine | UA Color ↑ | Blue Diapers (Alkaline Urine on Copper Fastenings) | |
| Urine | UA Hemoglobin ↑ | False + with Guaiac and Benzidine Tests | |
| Diazepam | Serum | Dihydroxyphenylalanine Screen Test + | Very Slight Purple Color Produced |
| Digoxin | Urine | 17 Ketosteroids ↓ | Slight Effect on Zimmerman Reaction in Vitro |
| Urine | 17 Hydroxy Corticosteroids ↑ | Moderate Effect with in Vitro Test | |
| Urine | Urobilin ↑ | Produces Yellow-Green Fluorescence | |
| Glucose | Whole Blood | Sedimentation Rate ↓ | High Blood Sugar Lowers Sedimentation Rate |
| Serum | Creatinine ↑ | Interferes with Jaffe Reaction | |
| Serum | Osmolality ↑ | Osmotically Active Constituent in Samples | |
| Serum | Uric Acid ↑ | Reducing Substance Reacts with Phosphotungstate | |
| Urine | Estriol ↓ | Interference with Gas Liquid Chromatography Method | |
| Urine | Osmolality ↑ | Osmotically Active Constituent in Samples | |
| Urine | Xylose Excretion ↑ | Interferes with Bromoaniline Procedure if Over 2g/100mL | |
| Urine | 17 Ketogenic Steroids ↓ | Interferes with Norymberski Reaction | |
| Urine | 17 Ketosteroids ↓ | Interferes with Zimmerman Reaction | |
| Heparin | Plasma | Ammonia ↑ | Contains Variable Amounts of Ammonium Salts |
| Plasma | Corticosteroids ↑ | If Contaminated by Impurities | |
| Plasma | Insulin ↓ | Effect in Heparinized Plasma and Serum | |
| Plasma | Insulin ↑ | Spuriously High Values Reported For Immunoassay | |
| Serum | Albumin ↑ | Promotes Binding of Haba Dye to Globulins | |
| Serum | Bromosulfophthalein Retention ↑ | Color Intensity ↑ in Serum, Wavelength Shifted | |
| Serum | Calcium ↓ | Interferes with EDTA and Fluorometric Methods | |
| Serum | Calcium ↑ | If Calcium Salt Used May Affect Result | |
| Serum | Creatine Phosphokinase ↓ | Reported Effect | |
| Serum | Hydroxybutyric Dehydrogenase ↓ | Significant Inactivation | |
| Serum | Lipoprotein Electrophor + | Alters Electrophoretic Pattern | |
| Serum | Phosphate ↑ | Phosphate Contamination of Heparin Reported | |
| Serum | Sodium ↑ | If Sodium Salt Used May Affect Result | |
| Serum | Thymol Turbidity ↑ | Affects Physico-Chem Properties Altering Turbidity | |
| Serum | Zinc Sulfate Turbidity ↑ | Affects Physico-Chem Properties | |
| Hydroxyzine | Urine | 17 Ketogenic Steroids ↑ | Interferes with Zimmerman Reaction |
| Urine | 17 Hydroxy Corticosteroids ↑ | Interferes with Porter-Silber Reaction | |
| Lidocaine | Cerebrospinal Fluid | Protein ↑ | Reacts with Folin-Ciocalteu Reagent |
| Magnesium Salts | Serum | Alkaline Phosphatase ↑ | Activators of Enzyme in Laboratory Procedures |
| Serum | Calcium ↑ | Measured as Calcium in Some Ethylenediaminetetraacetic Acid Procedures | |
| Mannitol | Serum | Phosphate ↓ | Inhibition of Color Development |
| Metronidazole | Urine | UA Color ↑ | Brown Color Probably Due to Metabolite |
| Nitrofurantoin | Urine | Alkaline Phosphatase ↓ | Interference with Determination Method |
| Urine | Lactic Dehydrogenase ↓ | Interference with Determination Method | |
| Urine | Sugar ↑ | Metabolites May Reduce Benedict’s, Yield False + | |
| Urine | UA Color ↑ | Brown, Yellow Color | |
| Phenols | Urine | Phenylketones + | Violet with Ferric Chloride, Nil With Phenistix |
| Urine | UA Color ↑ | Dark Green to Brownish Black on Standing | |
| Potassium | Serum | Calcium ↑ | Affects Flame Photometry if Poor Instrument |
| Serum | Sodium ↑ | Affects Flame Photometry if Poor Instrument | |
| Prochlorperazine | Urine | Phenylketones + | Light Purple with Ferric Chloride, Same with Phenistix |
| Urine | 17 Hydroxy Corticosteroids ↑ | Interferes with Porter-Silber Reaction | |
| Promethazine | Urine | Pregnancy Tests Negative | False Negative with Porter-Silber Reaction |
| Urine | Pregnancy Tests + | False + with Gravindex | |
| Urine | 17 Hydroxy Corticosteroids ↑ | Interference With Porter-Silber Reaction | |
| Urine | 5 Hydroxy Indoleacetic Acid ↓ | Interference with Nitrosonaphthol Methods | |
| Urine | UA Protein ↑ | False + with Labstix Due to High pH | |
| Sodium Chloride | Serum | Bilirubin ↓ | Inhibition of Diazo Test Reported |
| Vitamin A | Serum | Bilirubin ↑ | Interferes with Analysis |
| Serum | Cholesterol ↑ | Interferes with Zlatkis-Zak Reaction | |
| Serum | Direct Bilirubin ↑ | Interferes with Analysis | |
| Zinc | Urine | Magnesium ↑ | Measured by Fluorometric Method of Schachter |
| Zinc Salts | Serum | Alkaline Phosphatase ↓ | Inhibitors of Enzyme in Laboratory Procedures |
SGOT: Serum Glutamic-Oxalacetic Transaminase; SGPT: Serum Glutamic-Pyruvic Transaminase; UA: Urinalysis; G6PD: Glucose-6-Phosphate Dehydrogenase; RBC: Red Blood Cell; ODTC: Obtained During Test Conditions; + = Positive
METHODS
We conducted a retrospective chart review that was approved by our institutional review board. This review examined the medical records of 12 adult (age ≥ 18 years) burn patients with ≥ 20% total body surface area (TBSA) burns admitted to our facility from 2011 to 2012. Eligible patients required intensive insulin therapy (IIT) at admission and were part of an existing glycemic control database, which encompassed medical data from admission until the conclusion of IIT. Patient data was stratified into three groups: (a) 20 to 30%, (b) 31 to 60% and (c) 60 to 98% TBSA. Demographics and mortality data was collected. Daily multiple organ dysfunction score (MODS) was also included in our dataset. Medications dose, interval, and route of administration from the time of admission to discontinuation of intensive insulin therapy were recorded. Dosing in particular is included given the dose-dependent relationship of drug interferences on laboratory testing. The admission and intensive insulin therapy phases of burn care serve as high-risk time periods for these patients. Types of laboratory tests (i.e., complete blood count, basic metabolic panel, comprehensive metabolic panel, blood gases, and urinalysis) were also documented. Interfering substances were defined as compounds that cause inaccurate results for laboratory tests and were identified based on established clinical laboratory reference documentation. [14] Parametric data analysis was performed using MiniTab software (MiniTab, Inc., State College, PA). The 2-sample t-test compared independent means and repeated measures one-way analysis of variance (ANOVA) compared means between the three burn groups. Post-hoc pairwise comparisons via the Tukey’s HSD test were used for statistically significant ANOVA results. Tests for normality (Shapiro-Wilk) and nonparametric data analysis were performed using R statistical software (www.r-project.org). The Friedman test with repeated measures compared medians between the three burn groups.
RESULTS
Patients had a mean (SD) age of 37.9 (3.0) years, mean TBSA burn of 51.3% (9.3) and mean Multiple Organ Dysfunction Score (MODS) or 5.4 (0.2). Mortality was 8.3% (1/12 patients). Age, burn size, and MODS were similar (P > 0.05) between the three patient groups. Mean medications administered per day were 42.1 (9.5), and median medications administered were 49 with a daily range of 0 to 65 across all patients. A total of 666 potential interferences caused by medications administered were analyzed during intensive insulin therapy. Of these interferences, 261 were reported to have single discrete effects (e.g., increased glucose). Multiple potential interferences, 71.0% (475/666), were caused by more than one administered medication. Clinically significant drug interferences on laboratory testing were documented in two patients. Both cases involved high dose ascorbic acid during acute burn resuscitation. The interference resulted in significant and erroneous increases in (mean [SD] bias: 84.5 [25.2] mg/dL, P<0.001) point-of-care glucose meter results when compared to clinical laboratory methods unaffected by ascorbic acid therapy.
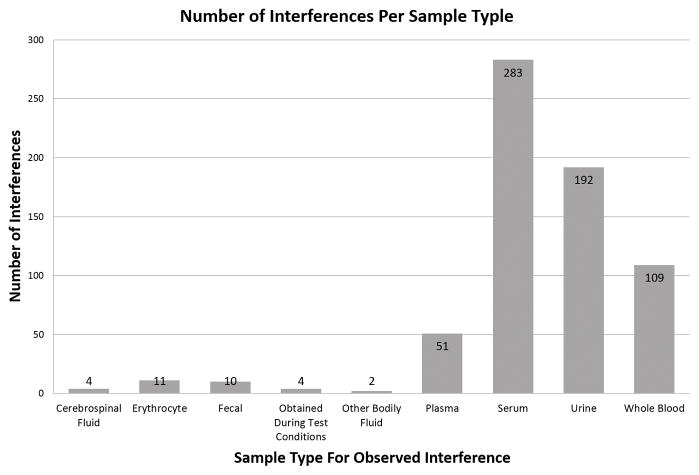
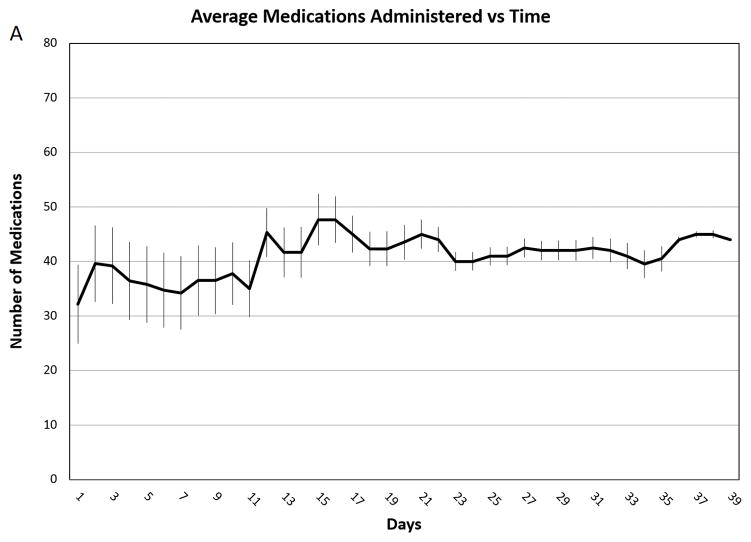
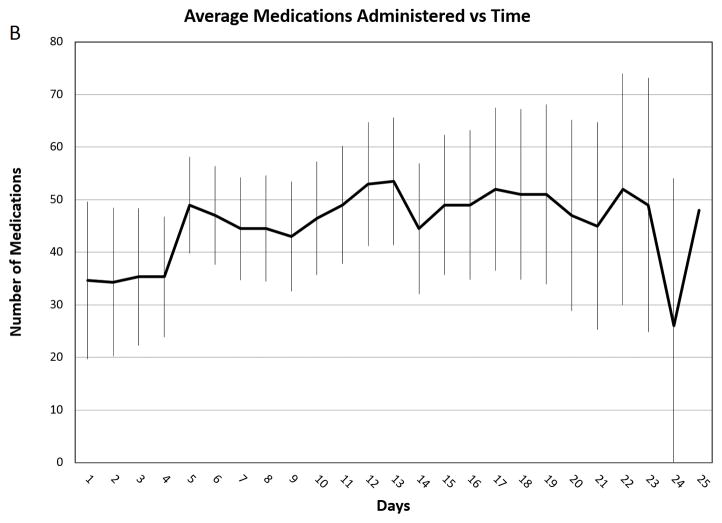
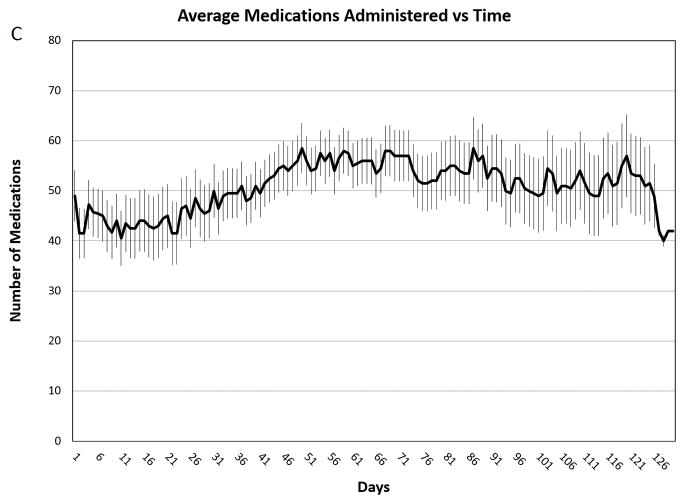
The most common sample types (i.e., serum, plasma, and urine specimens) were affected the most by drug interferences (Figure 1). When the mean medications per day were compared across the three different burn size groups (Figure 2), no statistically significant difference (P = 0.313) in mean medications per day relative to burn size was observed. Performing the Shapiro-Wilks test for normality revealed the data in both the 20 to 30% and 31 to 60% groups were normally distributed, however the data in the 61 to 98% group was slightly skewed. Nonparametric analysis revealed a statistically significant (P < 0.001) increase in median medications per day with respect to increase in burn size. Additionally, no statistically significant difference in the number of medications administered at either admission (P = 0.247) or the end (P = 0.483) of intensive insulin therapy was found.
Figure 1. Number of Interferences per Sample Type.
Illustrates the distribution of interferences amongst observed sample types. The most commonly used clinical samples types were found to contain the highest abundance of interferences. “Erythrocyte” refers to direct interference effects observed in red blood cells. “Obtained during test conditions” refer to interferences not seen clinically but observed in laboratory test conditions.
Figure 2. Average Medications Administered vs. Time.
Average medications administered per day throughout the course of intensive therapy. Panel A: Patients with 20–30% TBSA burns (n=5). Panel B: Patients with 31–60% TBSA burns (n=3). Panel C Patients with 61–98% TBSA burns (n=4). The error bars indicate standard deviations.
DISCUSSION
Treatment of burn patients requires a multitude medications and laboratory tests. Six hundred and sixty six potential interferences caused by medications administered at a mean rate of 42.1 (9.5) medications per day is a startling statistic. However, each of these medications serves a crucial purpose to ensure patients receive the best possible care. The potential impact of interfering substances on medical testing is well known in the field of laboratory medicine as shown by the volumes of reference material available to hospital laboratories and the rigorous validation of new medical tests through the United States Food and Drug Administration (FDA). Unfortunately, new drugs and laboratory tests are developed daily – making evaluation prior to clinical application for every drug and every test unrealistic.
Grouping patients into the 20 to 30%, 31 to 60%, and 61 to 98% TBSA stratifications allowed us to represent three at-risk populations. Intriguingly we found that this stratification of burn size did not reveal any significant differences in the mean number of medications administered per day. While we identified a significant difference in the median medications administered per day, nonparametric tests are more susceptible to Type I error (i.e., falsely accepting the alternative hypothesis when the null hypothesis is true). Mortality and disease severity have been shown to increase with burn size, thus one would assume clinical complications requiring medication therapy or treatment would also increase. [15] However, there are few studies investigating this interesting topic. Further studies with larger sample sizes should ultimately be conducted to further explore the relationship of medication frequency and its relation to patient outcomes.
Beyond the few examples of medications that result in dangerous erroneous laboratory measurements including the two cases encountered in this retrospective review, most manufacturer, FDA, and peer-reviewed literature reports mild to moderate effects by the majority of interfering substances which may be statistically significant, but perhaps not clinically significant. Those that are clinically significant can unfortunately put patients at risk for dangerous glycemic excursions and poor outcomes. Based solely on ascorbic acid interference, the observed glucometer bias of 84.5 mg/dL places patients at risk for hypoglycemia. [3] Additional medications being present in the patients system can further exacerbate this effect including hydroxycobalamin, which are increasingly being used in burn patients with suspected cyanide poisoning. [16] When taking into account the increasing number of medications released to market annually and the resulting increase in new medication interactions; the subject of medication interference is clearly an exponentially growing matter. [17]
Burn physicians, pharmacists, nurses, and laboratorians cannot maintain an ever-expanding list of complex pharmacological interactions relative to clinical laboratory analyses. The role of laboratory medicine in burn care could prove valuable and improve not only the quality of care, but also the safety of medical testing. Enhanced understanding of burn physiology by laboratory experts with close partnerships with burn critical care specialists enables quick recognition of potential interferences and development of diagnostic solutions in this high-risk population. At our institution, the burn care team works closely with our laboratory colleagues. This partnership has gone as far as to develop a rapid and dynamic system to obtain suspected interfering medications from the pharmacy to conduct real-time testing, confirm interferences, and quickly establish immediate solutions such as “priority one” plasma glucose testing in response to ascorbic acid interference on glucose meters. To date, the system has proven invaluable in identifying interfering substances including from the aforementioned high dose ascorbic acid therapy.
The reliance on human recognition of interfering substances is unfortunately not ideal. An innovative solution could lay in the use of electronic decision support tools. With the proliferation of electronic health record (EHR) and laboratory information systems (LIS), electronic decision support may provide unique opportunities to improve the safety of laboratory testing in high-risk patients. Medication administration records (MAR) within an EHR keep track of all the pertinent medication data. Laboratory test orders are sent via the EHR and are received by the LIS. Unfortunately, all three systems do not communicate effectively with one another and may not even display similar data. To this end, we recommend the creation of an automated tool within the EHR to act as a mediator between MAR and LIS that warns physicians about test results that may be affected by a currently administered medication.
While an upgrade to EHR systems would greatly enhance the quality of care for burn patients and other critically ill populations, we suggest going beyond EHR alerts. Ultimately in vitro diagnostics companies should develop laboratory tests that are robust to interfering substances. Similar endpoints have already been achieved for blood glucose monitoring systems (BGMS). Recent BGMS’s are designed to accurately measure blood glucose and automatically correct for interfering substances such as maltose, galactose, ascorbic acid, hematocrit, and high oxygen tension. [3,18,19] Enhanced performance of an autocorrecting BGMS during high dose ascorbic acid therapy, for example, was reported previously by our clinical studies in adult and pediatric burns and highlighting the value of robust biosensors for critical care. [3,20]
Limitations to our study include a small sample size of 12 patients. Additionally, the study was retrospective and at a single center. The use of medications and laboratory tests may vary between institutions. Lastly, our assessment focused from the time of admission until the conclusion of intensive insulin therapy.
CONCLUSIONS
The clinical impact of interfering substances on medical testing is well documented in laboratory medicine. These interferences have been shown to lead to erroneous measurements and impact patient care. Our study described the number of medications administered to burn patients and detailed potential laboratory test interferences that may lead to erroneous measurements. We recommend burn physicians work with laboratory personnel to identify potential interferences and define appropriate countermeasures. In parallel, laboratory personnel should work with burn care experts to improve their understanding of this unique critically ill population. Lastly, the development of intelligent electronic healthcare support tools capable of flagging potentially interfering drugs and autocorrecting biosensors could perhaps one day adjust the values in the presence of interfering substances with no intervention needed.
Supplementary Material
Acknowledgments
This study was supported in part by a National Heart Lung and Blood Institute (NHLBI) Emergency Medicine K12 career award and the National Center for Advancing Translational Sciences (award number: 5K12HL108964), National Institutes of Health (NIH), through grant number UL1 TR000002.
Footnotes
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
References
- 1.Ansermino M, Hemsley C. Intensive care management and control of infection. BMJ. 2004;329:220–3. doi: 10.1136/bmj.329.7459.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kroll MH, Elin RJ. Interference with clinical laboratory analyses. Clin Chem. 1994;40:1996–2005. [PubMed] [Google Scholar]
- 3.Tran NK, Godwin ZR, Bockhold JC, Passerini AG, Cheng J, Ingemason M. Clinical impact of sample interference on intensive insulin therapy in severely burned patients: a pilot study. J Burn Care Res. 35:72–9. doi: 10.1097/BCR.0b013e31829b3700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sartor Z, Kesey J, Dissanaike S. The Effects of Intravenous Vitamin C on Point-of-Care Glucose Monitoring. J Burn Care Res. 36:50–6. doi: 10.1097/BCR.0000000000000142. [DOI] [PubMed] [Google Scholar]
- 5.Baxter CR. Fluid volume and electrolyte changes of the early postburn period. Clin Plast Surg. 1974;1:693–703. [PubMed] [Google Scholar]
- 6.Baxter CR, Shires T. PHYSIOLOGICAL RESPONSE TO CRYSTALLOID RESUSCITATION OF SEVERE BURNS. Ann N Y Acad Sci. 1968;150:874–94. doi: 10.1111/j.1749-6632.1968.tb14738.x. [DOI] [PubMed] [Google Scholar]
- 7.Friedrich JB, Sullivan SR, Engrav LH, Round KA, Blayney CB, Carrougher GJ, et al. Is supra-Baxter resuscitation in burn patients a new phenomenon? Burns. 2004;30:464–6. doi: 10.1016/j.burns.2004.01.021. [DOI] [PubMed] [Google Scholar]
- 8.Pruitt BA. Protection from excessive resuscitation: “Pushing the pendulum back. J Trauma - Inj Infect Crit Care. 2000;49:567–8. doi: 10.1097/00005373-200009000-00030. [DOI] [PubMed] [Google Scholar]
- 9.Engrav LH, Colescott PL, Kemalyan N, Heimbach DM, Gibran NS, Solem LD, et al. A biopsy of the use of the Baxter formula to resuscitate burns or do we do it like Charlie did it? J Burn Care Rehabil. 2000;21:91–5. doi: 10.1097/00004630-200021020-00002. [DOI] [PubMed] [Google Scholar]
- 10.Horton JW, White DJ, Maass DL, Hybki DP, Haudek S, Giroir B. Antioxidant vitamin therapy alters burn trauma-mediated cardiac NF-kappaB activation and cardiomyocyte cytokine secretion. J Trauma. 2001;50:397–406. doi: 10.1097/00005373-200103000-00002. discussion 407–8. [DOI] [PubMed] [Google Scholar]
- 11.Matsuda T, Tanaka H, Shimazaki S, Matsuda H, Abcarian H, Reyes H, et al. High-dose vitamin C therapy for extensive deep dermal burns. Burns. 1992;18:127–31. doi: 10.1016/0305-4179(92)90009-j. [DOI] [PubMed] [Google Scholar]
- 12.Tang Z, Du X, Louie RF, Kost GJ. Effects of drugs on glucose measurements with handheld glucose meters and a portable glucose analyzer. Am J Clin Pathol. 2000;113:75–86. doi: 10.1309/QAW1-X5XW-BVRQ-5LKQ. [DOI] [PubMed] [Google Scholar]
- 13.Abarca J, Colon LR, Wang VS, Malone DC, Murphy JE, Armstrong EP. Evaluation of the performance of drug-drug interaction screening software in community and hospital pharmacies. J Manag Care Pharm. 2006;12:383–9. doi: 10.18553/jmcp.2006.12.5.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Young DS, Pestaner LC, Thomas DW. Clinical Effects of Drugs on Tests. 1972:18. [PubMed] [Google Scholar]
- 15.Smith DL, Cairns BA, Ramadan F, Dalston JS, Fakhry SM, Rutledge R, et al. Effect of inhalation injury, burn size, and age on mortality: a study of 1447 consecutive burn patients. J Trauma. 1994;37:655–9. doi: 10.1097/00005373-199410000-00021. [DOI] [PubMed] [Google Scholar]
- 16.Carlsson CJ, Hansen HE, Hilsted L, Malm J, Ødum L, Szecsi PB. An evaluation of the interference of hydroxycobalamin with chemistry and co-oximetry tests on nine commonly used instruments. Scand J Clin Lab Invest. 2011;71:378–86. doi: 10.3109/00365513.2011.573573. [DOI] [PubMed] [Google Scholar]
- 17.Ansari J. Drug interaction and pharmacist. J Young Pharm. 2010;2:326–31. doi: 10.4103/0975-1483.66807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tran NK, Promptmas C, Kost GJ, Cook KW, Lehmann C, Schoeff LWR. Biosensors, miniaturization, and noninvasive techniques. Clin diagnostic Technol Total Test Process preanalytical, Anal post-analytical phases. 2006:145–84. [Google Scholar]
- 19.Rao LV, Jakubiak F, Sidwell JS, Winkelman JW, Snyder ML. Accuracy evaluation of a new glucometer with automated hematocrit measurement and correction. Clin Chim Acta. 2005;356:178–83. doi: 10.1016/j.cccn.2005.01.027. [DOI] [PubMed] [Google Scholar]
- 20.Tran NK, Godwin ZR, Steele AN, Wade C, Wolf S, Palmieri Tina L. Clinical Impact of Accurate Glucose Monitoring for Tight Glycemic Control in Severely Burned Children. Crit Care. 2015 doi: 10.1097/PCC.0000000000000877. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.