Structured Abstract
Objective
To compare bariatric surgery vs. intensive medical weight management (MWM) in patients with type 2 diabetes (T2DM) who do not meet current NIH criteria for bariatric surgery.
To assess whether the soluble form of receptor for advanced glycation endproducts (sRAGE) is a biomarker to identify patients most likely to benefit from surgery.
Summary Background Data
There are few studies comparing surgery to MWM for patients with T2DM and BMI < 35.
Methods
57 patients with T2DM and BMI 30–35 who otherwise met criteria for bariatric surgery were randomized to MWM vs. surgery (bypass, sleeve or band, based on patient preference). The primary outcomes assessed at 6 months were change in insulin resistance (HOMA-IR) and diabetes remission. Secondary outcomes included changes in HbA1c, weight, and sRAGE.
Results
The surgery group had improved HOMA-IR (−4.6 vs. +1.6; p=0.0004) and higher diabetes remission (65% vs. 0%, p<0.0001) than the MWM group at 6 months. Compared to MWM, the surgery group had lower HbA1c (6.2 vs. 7.8, p=0.002), lower fasting glucose (99.5 vs. 157; p=0.0068) and fewer T2DM medication requirements (20% vs. 88%; p<0.0001) at 6 months. The surgery group lost more weight (7.0 BMI decrease vs. 1.0 BMI decrease, p<0.0001). Higher baseline sRAGE was associated with better weight loss outcomes (r=−0.641; p=0.046). There were no mortalities.
Conclusions
Surgery was very effective short-term in patients with T2DM and BMI 30–35. Baseline sRAGE may predict patients most likely to benefit from surgery. These findings need to be confirmed with larger studies.
INTRODUCTION
Up to 78% of patients with type 2 diabetes mellitus (T2DM) may experience diabetes remission within two years after bariatric surgery.1 Currently, only patients with T2DM and body mass index (BMI) above 35 kg/m2 are eligible for bariatric surgery. This is based on the 1991 National Institutes of Health (NIH) Guidelines and has been endorsed by the Center for Medicare and Medicaid Services.2 Patients with T2DM and BMI < 35 are primarily offered intensive medical weight management (MWM), including pharmacotherapy and nonsurgical weight loss strategies. Millions of patients with T2DM have BMI < 35—yet metabolic surgery is not an option for them.3
There is emerging evidence supporting the use of bariatric surgery to treat diabetes in less obese (BMI < 35) patients. However, there are very few randomized trials. The Agency for Healthcare Research and Quality (AHRQ) recently identified this area as a research priority for comparative effectiveness research.4 The NIH is unlikely to change the bariatric surgery guidelines for patients with T2DM without additional evidence to support such a change.5 There is also an overall lack of treatment data in underrepresented minorities (especially Hispanics and non-Hispanic African-Americans) with T2DM, who are disproportionately affected by diabetes-related complications and mortality. Our municipal health care system serves a substantial number of these “hard-to-reach” minorities.
The receptor for advanced glycation end-products (RAGE) binds multiple ligand families linked to hyperglycemia. Activation of RAGE plays a major role in the pathogenesis of diabetic vascular complications via activation of the nuclear factor κ β pathway.6 Recent data indicate that mice devoid of RAGE who are fed high fat diet are protected from diet-induced obesity and that the treatment of diabetic mice with a soluble form of RAGE (sRAGE) results in significantly reduced body weight gain vs. vehicle-treated animals.7 Interestingly, sRAGE already circulate in human plasma. sRAGE acts as a decoy to prevent an interaction between advanced glycation end-products and RAGE and subsequent RAGE ligand-mediated effects on obesity and diabetes complications.8 Therefore, levels of sRAGE may be innate biomarkers of vulnerability to obesity and diabetes and/or their severity.9
The purpose of this study was two-fold: 1) to conduct a pilot randomized trial to compare bariatric surgery to MWM in patients with T2DM and BMI 30–35 who otherwise met NIH criteria for surgery, and 2) to assess the role of sRAGE as a biomarker for predictor of success after surgery. Due to the fact that insurance companies typically cover bariatric surgery in patients with T2DM and BMI > 35, we partnered with our municipal healthcare system’s primary insurer, who agreed to cover the costs of the surgery as part of this research project.
METHODS
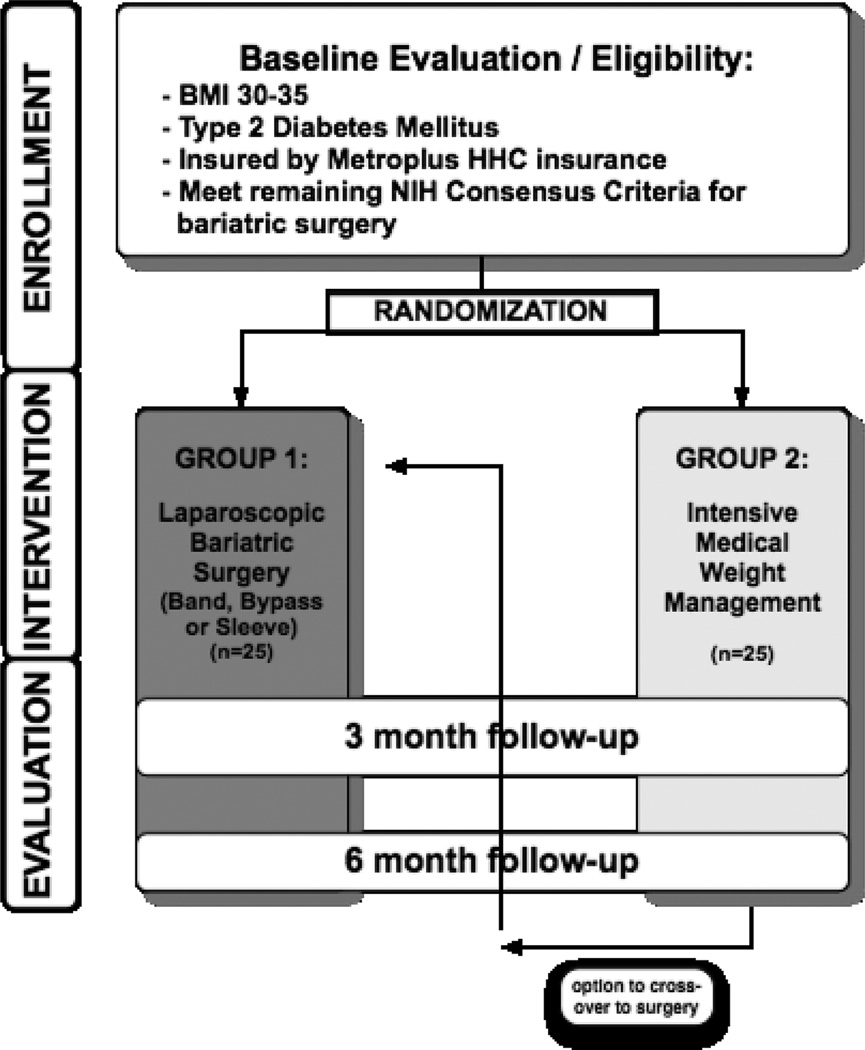
We conducted a randomized controlled trial among patients with T2DM and BMI 30–35 who were otherwise eligible for bariatric surgery by NIH criteria, specifically: (1) overweight for at least 5 years, (2) failure to lose weight with non-surgical means, (3) absence of medical or psychological contraindications, (4) patient understanding of the procedure and its risks, and (5) strong motivation to comply with the post-surgical regimen. Patients were excluded if they were deemed unable to comply with the study protocol (either self-selected or by indicating during screening that s/he could not complete all requested tasks), participation in other obesity- or diabetes-related clinical trials, or diagnosis of cognitive dysfunction or significant psychiatric comorbidity.
Our municipal health care system is the New York City Health and Hospitals Corporation (HHC). HHC is the public safety-net healthcare system of New York City. It is the largest municipal health care system in the US, serving over 1.4 million patients, including over 475,000 uninsured city residents. HHC operates its own 400,000 member health plan—MetroPlus, which offers New York State sponsored Medicaid-Managed care to those who have or are eligible for Medicaid and live in Brooklyn, Queens, Manhattan and the Bronx. MetroPlus agreed to cover the costs of surgery and any associated complications of patients enrolled in this study.
Recruitment strategies included utilizing electronic medical records to identify patients with diabetes based on ICD-9 codes, notifying medical physicians of the study, sending out letters to all patients on various diabetes registries within our municipal health system, and partnering with MetroPlus to identify eligible patients.
Patients were screened for eligibility by study staff, provided informed consent by language-concordant Research Assistants (RAs), and then randomized to one of the two study arms (Fig. 1). Patients were randomized by RAs calling a central phone number to receive allocation; the allocation sequence was generated in advance and stratified to ensure balance of patients with BMI 30–35 in each of the arms. All data was collected using standard study templates, based on the CONSORT statement.10 Institutional Review Board (IRB) approval was obtained prior to enrolling the first patient.
Figure 1.
Trial Flow
Intensive Medical Weight Management (MWM) Protocol
The MWM protocol was based on successful models of lifestyle counseling previously published in the medical literature.11–14 These trials utilized intensive lifestyle interventions, including frequent group and individual sessions focusing on nutrition and physical activity counseling. For our short-term study, the weight loss goal was 5% of initial body weight at 6 months.
MWM sessions were led by a bilingual weight loss clinician with an expertise in diabetes education. Sessions were held weekly for the first month and then biweekly. In these 30-minute sessions, the clinician offered culturally tailored, patient-specific counseling on diet, physical activity, self-monitoring, and goal setting. The visits included a review of home glucose data and adjustment of diabetes medications. In addition, participants were provided with pedometers to track their progress, with a goal of 150 min/week of low-impact physical activity by 6 months.
Patients randomized to the MWM arm were given the option to cross over to the surgical arm after completing 6 months of MWM. The cross-over group existed to ensure compliance with the MWM group, as a previous randomized study conducted at our institution (looking at a different research question involving MWM) suffered from high drop-out/non-compliance in the MWM arm.15 A minimum of 2% weight loss was required for all MWM participants in order to proceed with the surgery (to ensure maximum compliance with the MWM arm).
Bariatric Surgery Protocol
Patients randomized to surgery underwent bypass, band or sleeve gastrectomy based on patient preference (drawing upon information learned in the monthly bariatric surgery information seminar and during the surgeon consultation). All patients underwent thorough evaluation by a surgeon, internist, nutritionist, and psychologist, and then completed a liquid protein diet for two weeks prior to surgery to decrease hepatomegaly.
Laparoscopic gastric bypass was performed with a 150 cm Roux limb and 100 cm biliopancreatic limb. Laparoscopic adjustable gastric banding was performed utilizing the pars flaccida technique and the Lap-Band AP-Standard (California, Inamed). Sleeve gastrectomy was created over a 40Fr bougie, approximately 5–7 cm proximal to the pylorus, utilizing bioabsorbable buttressing material on the staple line.16 Postoperative dietary guidelines were based on the ASMBS Allied Health Nutritional Guidelines for the Surgical Weight Loss Patient.17 Patients were maintained on clear liquids for 48 hours postoperatively, then advanced to full liquid diet (including low-fat, low sugar, protein-rich shakes) for 2 weeks, followed by pureed diet for 2 weeks and then transitioned to regular diet. Patients were seen postoperatively at 2 weeks, 4 weeks and then monthly for the duration of the study. Band adjustments were done percutaneously in the clinic according to a commonly used algorithm based on hunger and weight loss.18
Definitions and study-related measures
T2DM was defined based on the American Diabetes Association (ADA) criteria: (1) fasting glucose ≥ 126 mg/dL or (2) glucose ≥ 200 at 120 minutes after 75 g oral glucose load or (3) HbA1c ≥ 6.5%.19 Diabetes remission was defined as no longer meeting the ADA criteria for T2DM, without the use of diabetes medications.
The following study-related measures were collected at baseline and 6 months follow-up: insulin, glucose, glycated hemoglobin (HbA1c), weight, BMI, blood pressure, waist circumference, fasting lipids, blood pressure and sRAGE. An oral glucose tolerance test (OGTT) was performed at 6 months on all patients, when not contraindicated (if fasting glucose was > 126 mg/dl, then the OGTT test was not performed). The patient was instructed to fast overnight and then was given a 75g oral glucose load. Glucose levels were tested 120 minutes later. The homeostatic model of insulin resistance (HOMA-IR) was calculated as: [(glucose mg/dl × insulin)/405]. Remission of diabetes and total and % excess weight loss (%EWL) were assessed at 6 months. Excess weight loss was calculated based on the Robinson formula for ideal body weight.20 Plasma sRAGE levels were assayed using enzyme-linked immunosorbent assay (ELISA) kits in accordance with the manufacturer’s protocol (R&D Systems Quantikine Immunoassay Minneapolis, MN). The primary outcomes assessed at 6 months were (1) change in insulin resistance (HOMA-IR) and (2) diabetes remission.
Statistics
We calculated that 50 patients (25 in each arm) would constitute an adequately powered study to study both primary outcomes. The sample size for the primary outcome of change in HOMA-IR was assessed by calculating the size of detectable standard deviation units (SDU). The SDU corresponds to a beta coefficient in a regression model when we assume the standard normal deviate, i.e. N(0,1). Using an alpha error of 5%, we calculated that this study would have 80% power to detect an SDU of 0.81, corresponding to nearly 1/5th of the range of values. If we assume a similar distribution of HOMA IR found in previous studies,21 an SDU of 0.81 corresponds to a mean change in HOMA IR of 0.67. The sample size for the second primary outcome of diabetes remission was assessed by calculating the size of detectable difference in a binomial proportion. Using an alpha error of 5% and a diabetes remission rate of 13% in the MWM arm of a previous study,21 we calculated that this study would have 80% power to detect a remission rate of 52% (i.e. absolute change of 39%) in the bariatric surgery arm.
All study aims were tested based on a two-tailed significance level of 0.05. All analyses were intention-to-treat, i.e. patients were analyzed according to the group they are assigned at randomization. SAS version 9.1 (SAS Institute, Cary, NC, USA) was used for all data manipulations and statistical analysis. Demographic data was compared utilizing two-sample t-test or Fisher’s exact test, when applicable. Outcomes were compared using two-sample t-test, Fisher’s exact test, and ANOVA test, when applicable. Scatter plots and Pearson’s correlation tests were used to evaluate the sRAGE biomarker as a predictor of success.
Results
A total of 57 patients with T2DM and BMI 30–35 who otherwise met criteria for bariatric surgery were randomized to MWM (n=28) or surgery (n=29; bypass, sleeve or band, based on patient preference). Demographics are shown in Table 1. The patients randomized to surgery were slightly younger. The majority of the patients were female Hispanic or non-Hispanic African-American, consistent with the patient population at our urban safety-net institution. Mean baseline HbA1c was 7.8 and 36% of patients were using insulin. Sleeve was the most popular procedure chosen by patients.
Table 1.
Baseline Data
| MWM N=28 |
Surgery N=29 |
p-value | |
|---|---|---|---|
| Age (years) | 53.9 (8.4) | 46.8 (8.1) | 0.0020 |
| Female | 79% (n=22) | 79% (n=23) | 0.999 |
| Race | |||
| Hispanic | 89% (n=25) | 86% (n=25) | 0.612 |
| African American | 4% (n=1) | 7% (n=2) | |
| BMI (kg/m2) | 32.4 (1.8) | 32.8 (1.7) | 0.331 |
| Waist circumference (cm) | 106.7 (7.7) | 106.3 (10.1) | 0.862 |
| HOMA-IR | 3.5 (2.9) | 5.1 (3.4) | 0.076 |
| HbA1c | 7.9 (1.3) | 7.7 (1.4) | 0.622 |
| Requiring insulin | 39% (n=11) | 34% (n=10) | 0.084 |
| Triglycerides | 156.5 (69.1) | 196.9 (188.2) | 0.287 |
| HDL | 46.4 (13.2) | 47.2 (15.5) | 0.851 |
| LDL | 116.1 (55.9) | 101.8 (88.2) | 0.261 |
| CHOL | 193.9 (66.6) | 193.4 (61.7) | 0.977 |
| Systolic BP | 129.1 (19.0) | 126.4 (16.6) | 0.577 |
| Diastolic BP | 75.3 (8.1) | 77.0 (13.3) | 0.596 |
| Type of surgery selected | |||
| None | 100% (n=28) | 3% (n=1) | |
| LAGB | - | 17% (n=5) | NA |
| LSG | - | 55% (n=16) | |
| RYGB | - | 24% (n=7) | |
MWM – medical weight management
BMI – body mass index
HOMA-IR – homeostatic model of insulin resistance
HBA1c – glycated hemoglobin
LAGB – laparoscopic adjustable gastric banding
LSG – laparoscopic sleeve gastrectomy
RYGB – laparoscopic Roux-en Y gastric bypass
Continuous data are presented as mean (SD) and were compared using a two-sample t-test; percentages were compared using Fisher’s exact test.
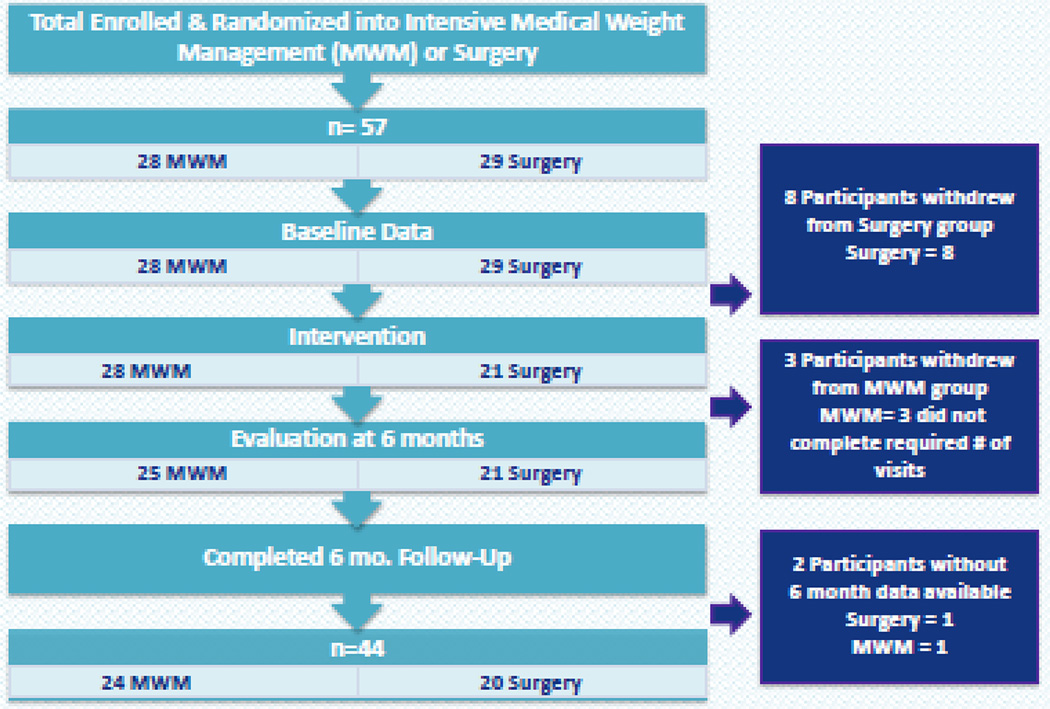
Ultimately, 44 patients (23% dropout rate) completed 6 month follow-up (Fig. 2). Mean number of MWM visits for patients in the MWM arm was 11.5. Six month follow-up in the surgery group was 95% (one patient moved out-of-state). The surgery group had significantly improved HOMA-IR (−4.6 vs. +1.6; p=0.0004) and higher diabetes remission (65% vs. 0%, p<0.0001) than the MWM group at 6 months (Table 2). The surgery group also had significantly lower HbA1c (6.2 vs. 7.8, p=0.002), lower fasting glucose (99.5 vs. 157; p=0.0068), lower glucose after oral glucose load (130.2 vs. 306, p<0.0001) and fewer T2DM medication requirements (20% vs. 88%; p<0.0001) at 6 months. The surgery group had significantly better weight loss outcomes (7.0 BMI decrease vs. 1.0 BMI decrease, p<0.0001) and change in waist circumference (−16.1 cm vs. −1.8 cm, p<0.0001) compared to the MWM group at 6 months (Table 3). There was no significant difference in lipid panels or blood pressure measurements, probably due to the fact that most patients had relatively normal lipid profiles and were normotensive at baseline.
Figure 2.
Patient Flow
Table 2.
Primary outcome measures for MWM versus bariatric surgery at 6 months
| MWM N=24 |
Surgery N=20 |
p-value | ||
|---|---|---|---|---|
| HOMA-IR | Pre | 3.1 (2.6) | 6.5 (3.9) | 0.0032 |
| Post | 4.7 (4.9) | 1.8 (1.2) | 0.013 | |
| Change | +1.6 (4.9) | −4.6 (4.1) | 0.0004 | |
| Diabetes Remission | Yes | 0% (n=0) | 65% (n=13) | <0.0001 |
| No | 100% (n=24) | 35% (n=7) | ||
| HbA1c | Pre | 7.7 (1.0) | 7.4 (1.2) | 0.274 |
| Post | 7.8 (1.7) | 6.2 (0.9) | 0.0002 | |
| Change | +0.1 (1.5) | −1.2 (1.1) | 0.0027 | |
| Fasting glucose | Pre | 143.6 (46.9) | 149.6 (45.5) | 0.675 |
| Post | 156.9 (91.3) | 99.5 (28.0) | 0.0068 | |
| Change | +13.3 (77.8) | −50.1 (44.4) | 0.0017 | |
| Glucose after OGTT | Post | 306.3 (108.1) | 130.2 (78.0) | <0.0001 |
| Requiring T2DM meds | Yes | 88% (n=21) | 20% (n=4) | <0.0001 |
| No | 12% (n=3) | 80% (n=16) | ||
MWM – medical weight management
HOMA-IR – homeostatic model of insulin resistance
HBA1c – glycated hemoglobin
OGTT – oral glucose tolerance test
T2DM – type 2 diabetes
Continuous data are presented as mean (SD) and were compared using a two-sample t-test Diabetes medication and diabetes remission were compared using a Fisher’s exact test
Table 3.
Secondary outcome measures for MWM versus surgery at 6 months
| MWM N=24 |
Surgery N=20 |
p-value | ||
|---|---|---|---|---|
| Weight (lbs) | Pre | 184.5 (23.8) | 180.7 (17.0) | 0.551 |
| Post | 180.5 (29.2) | 142.5 (22.5) | <0.0001 | |
| Change | −4.0 (9.2) | −38.2 (13.8) | <0.0001 | |
| BMI (kg/m2) | Pre | 32.4 (1.8) | 32.8 (1.6) | 0.421 |
| Post | 31.4 (2.6) | 25.9 (2.5) | <0.0001 | |
| Change | −1.0 (1.5) | −7.0 (2.6) | <0.0001 | |
| %EWL | Post | 7.4% (12.6) | 60.0% (21.1) | <0.0001 |
| Waist circumference (cm) | Pre | 106.8 (7.8) | 107.5 (9.3) | 0.785 |
| Post | 104.9 (9.4) | 91.4 (8.5) | <0.0001 | |
| Change | −1.8 (5.1) | −16.1 (8.9) | <0.0001 | |
| Triglycerides | Pre | 151.9 (67.5) | 175.1 (99.6) | 0.366 |
| Post | 130.0 (60.4) | 123.0 (58.8) | 0.697 | |
| Change | −21.9 (79.0) | −52.1 (96.7) | 0.260 | |
| HDL | Pre | 47.4 (13.5) | 48.4 (17.9) | 0.845 |
| Post | 53.5 (29.6) | 50.9 (12.3) | 0.703 | |
| Change | +6.0 (27.6) | +2.6 (12.5) | 0.582 | |
| LDL | Pre | 112.2 (51.2) | 104.4 (36.4) | 0.579 |
| Post | 102.0 (32.1) | 124.4 (41.8) | 0.054 | |
| Change | −10.2 (52.5) | +20.0 (44.5) | 0.053 | |
| CHOL | Pre | 190.0 (61.6) | 191.5 (45.9) | 0.931 |
| Post | 170.5 (36.3) | 203.3 (42.6) | 0.0094 | |
| Change | −19.5 (58.3) | +11.8 (54.8) | 0.080 | |
| Systolic BP | Pre | 129.0 (19.4) | 127.4 (17.5) | 0.797 |
| Post | 131.4 (18.7) | 127.1 (19.3) | 0.467 | |
| Change | +2.5 (19.1) | −0.4 (19.7) | 0.640 | |
| Diastolic BP | Pre | 75.6 (8.6) | 78.7 (13.6) | 0.405 |
| Post | 75.3 (11.4) | 78.7 (11.2) | 0.348 | |
| Change | −0.2 (12.3) | +0.1 (10.7) | 0.941 | |
MWM – medical weight management
BMI – body mass index
%EWL - % excess weight loss
Continuous data are presented as mean (SD) and were compared using a two-sample t-test
When stratifying by surgery type, LSG had the highest rate of diabetes remission, however this analysis was likely limited by the small sample size (Table 4). For instance, more patients who underwent bypass (3/6) were on insulin preoperatively compared to sleeve (1/11). When stratifying by baseline medication type, patients who were not using insulin at baseline had 80% remission after surgery, while those on insulin experienced 20% remission after surgery (p=0.015).
Table 4.
Primary outcome measures for surgery patients (N=20 with 6-month data) by type of surgery
| LAGB N=3 |
LSG N=11 |
RYGB N=6 |
p-value | ||
|---|---|---|---|---|---|
| HOMA-IR | Pre | 8.0 (3.7) | 6.5 (4.7) | 5.3 (2.7) | 0.705 |
| Post | 2.1 (1.2) | 1.4 (1.2) | 2.4 (1.2) | 0.446 | |
| Change | −5.8 (2.8) | −5.1 (4.9) | −2.9 (3.6) | 0.634 | |
| Diabetes Remission | Yes | 33% (n=1) | 91% (n=10) | 33% (n=2) | 0.025 |
| No | 67% (n=2) | 9% (n=1) | 67% (n=4) | ||
| Fasting glucose | Pre | 123.3 (30.6) | 159.1 (46.9) | 145.2 (49.7) | 0.489 |
| Post | 94.7 (15.9) | 89.7 (15.9) | 119.8 (40.7) | 0.096 | |
| Change | −28.7 (16.1) | −69.4 (45.9) | −25.3 (36.7) | 0.093 | |
| Glucose after OGTT | Post | 161.0 (41.8) | 104.0 (39.6) | 154.0 (122.4) | 0.383 |
| Requiring T2DM meds | Yes | 67% (n=2) | 0% (n=0) | 33% (n=2) | 0.016 |
| No | 33% (n=1) | 100% (n=11) | 67% (n=4) | ||
HOMA-IR – homeostatic model of insulin resistance
OGTT – oral glucose tolerance test
T2DM – type 2 diabetes
LAGB – laparoscopic adjustable gastric banding
LSG – laparoscopic sleeve gastrectomy
RYGB – laparoscopic Roux-en Y gastric bypass
Continuous data are presented as mean (SD) and were compared using a ANOVA test Diabetes medication and diabetes remission were compared using Fisher’s exact tests
Seven patients crossed over to surgery after completing the MWM arm. None of these experienced diabetes remission after the MWM intervention. However, after crossover to surgery, 3 patients (43%) experienced diabetes remission. These crossover patients had more significant BMI decrease (−6.4 vs. −1.3; p=0.0085) and increased %EWL (60.3% vs. 10.8%, p=0.004) after surgery. The change in HBA1c was not significant between the 2 groups in HBA1c (−0.6 vs. −0.1; p=0.722), however the mean post-surgery follow-up was less than 6 months.
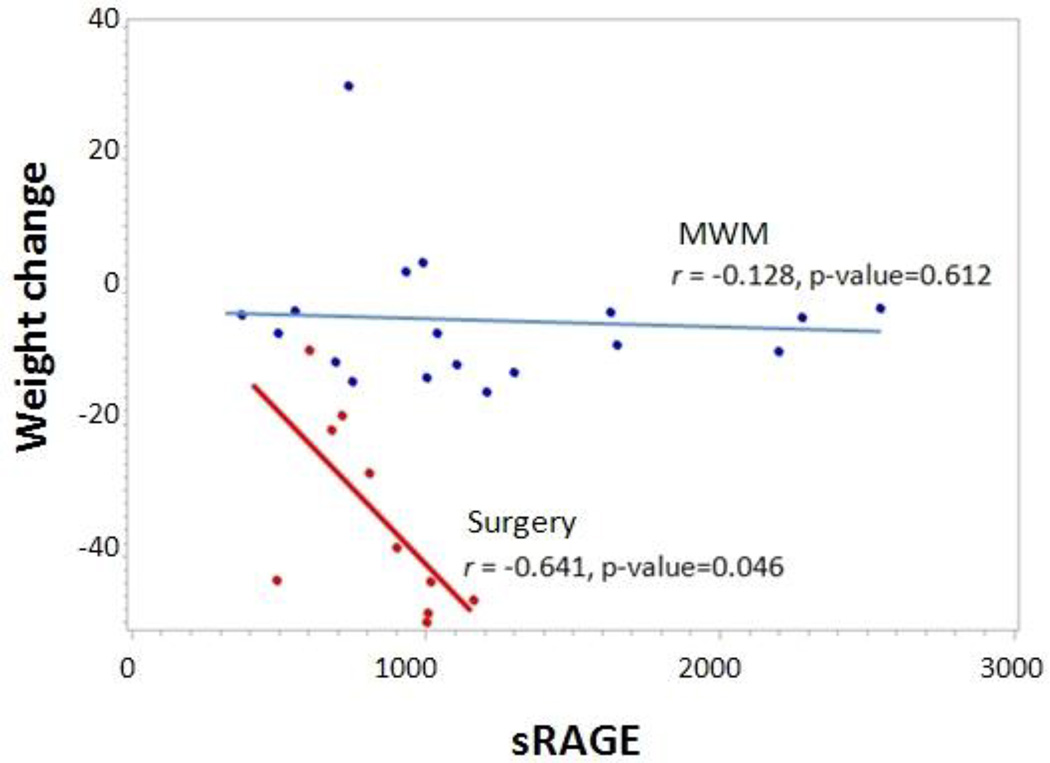
There was no association between pre- and post-treatment sRAGE with pre- and post-treatment characteristics, supporting the possibility of sRAGE as an independent biomarker (Table 5). Higher baseline sRAGE was associated with better weight loss outcomes, p=0.046 (Fig. 3). In the surgery group, mean sRAGE increased from 812 to 1044 pg/ml, but this was not significant (p=0.319). In the MWM group, sRAGE decreased from 1162 to 995 pg/ml (p=0.403).
Table 5.
No association between pre- and post-treatment sRAGE with pre- and post-treatment patient characteristics
| Pre-treatment N=38 |
Post-treatment N=22 |
|||
|---|---|---|---|---|
| r | p-value | r | p-value | |
| Weight | −0.057 | 0.740 | 0.224 | 0.316 |
| BMI | −0.014 | 0.936 | 0.110 | 0.626 |
| Waist circumference | 0.055 | 0.745 | 0.200 | 0.398 |
| Fasting glucose | −0.174 | 0.296 | 0.060 | 0.792 |
| Insulin | −0.231 | 0.181 | −0.035 | 0.877 |
| HbA1c | −0.021 | 0.902 | 0.324 | 0.141 |
BMI – body mass index
HBA1c – glycated hemoglobin
Figure 3.
Baseline sRAGE as predictor of weight loss after surgery
There were no complications in the MWM arm. Hospitalizations unrelated to MWM were not tracked in this study. There were no mortalities or life-threatening complications in the surgery arm. There was one readmission with 30 days for dehydration (resolved with intravenous fluids and anti-emetics) and there was one late readmission (>30 days) for a trocar site abscess (resolved with percutaneous drainage and antibiotics).
DISCUSSION
There are very few randomized controlled trials (RCT) focusing on the role of bariatric surgery in patients with T2DM and BMI 30–35. There is also very little data in Hispanics and non-Hispanic African-Americans with T2DM, who are disproportionately affected by diabetes and diabetes-related complications. The AHRQ has recognized this area as a critical research gap. There is an urgent need for large-scale RCTs comparing the effectiveness of bariatric surgery to MWM for patients with T2DM and BMI < 35. The NIH is unlikely to change the guidelines without such data. There is also a need to determine which patients would benefit most from surgical intervention. Our pilot study found that surgery was very effective in the short-term in mainly Hispanic and non-Hispanic African-Americans with T2DM and BMI 30–35 (65% diabetes remission rate); we also found that higher baseline sRAGE correlated with improved weight-loss outcomes after surgery.
Our data are in accordance with previous studies in this area. We recently conducted a systematic review and meta-analysis of the existing data regarding bariatric surgery in patients with T2DM and BMI < 35 (n=1389) and calculated a 55% estimated diabetes remission rate at 12 months.22 However, the bulk of the source data in this review were retrospective data. Most of the existing RCTs contain patients with BMI >35.21, 23–25. Two RCTs have been conducted in patients with T2DM and BMI < 35, however neither used MWM as the comparator arm.26, 27 This is one of the first studies focusing on BMI 30–35 using MWM as the comparator arm.
This pilot study may help design future larger-scale trials in this arena. Recruitment was a significant challenge at the outset, primarily due to the fact that most patients with BMI 3035 did not consider themselves obese. Strategies to enhance patient recruitment included redesigning flyers to focus on diabetes remission (instead of weight loss), sending out flyers to patients on the diabetes registry in our system, and initiating group information seminars specifically tailored to patients with T2DM and BMI 30–35. A population-based recruitment strategy may be the most pragmatic.28 Also, the sleeve was the most popular procedure chosen by patients randomized to surgery (mainly due to the lower risk profile compared to the bypass but greater efficacy than the band). Future studies should consider utilizing sleeve as the comparator. Future studies should also take into account the 23% dropout rate experienced in our study.
There are very little data about the clinical utility of sRAGE as a biomarker. sRAGE prevents activation of RAGE by advanced glycation endproducts. A recent study of 85 morbidly obese patients (who did not have T2DM) revealed that patients with morbid obesity have lower sRAGE compared with non-obese controls, and that sRAGE increases after bariatric surgery.8 Our study also found an increase in sRAGE after bariatric surgery, however it was not statistically significant, probably due to the limited sample size. Baseline sRAGE may have the potential to be a biomarker of diabetes.9 We did find that baseline sRAGE correlated with weight loss after bariatric surgery. This implies that baseline sRAGE levels may be useful to predict T2DM patients most likely to benefit from surgical intervention for weight loss. This finding needs to be corroborated with larger-scale studies with longer follow-up.
Limitations of this study include the small sample size and the short-term duration (6 months). The original intent of this study was to conduct a pilot RCT to establish the groundwork for a larger longer-term study. We plan to follow this patient cohort and re-analyze the HOMA-IR and diabetes remission rate at one and two years. Due the small sample size, we were unable to reliably compare band, bypass and sleeve on diabetes outcomes. Furthermore, once patients were randomized to surgery, the actual procedure selection was determined by the patient (i.e. patients were not randomized to a particular procedure). Therefore, our finding that the sleeve had the highest diabetes remission rate is likely due to small sample size and/or selection bias (more severe T2DM patients had bypass compared to sleeve).
Another potential criticism of this study is that the comparator arm was MWM, not “intensive diabetes management,” specifically that the comparator arm did not titrate diabetes medications to achieve better glycemic control. However the aims of this study were to evaluate indices of insulin resistance (HOMA-IR) and T2DM remission, not diabetes control. Aggressively titrating diabetes medications is unlikely to improve HOMA-IR or induce T2DM remission. Weight loss and lifestyle modifications are the primary methods of improving insulin resistance and inducing T2DM remission. Our MWM arm could have perhaps integrated more extensive lifestyle changes (e.g., using validated tools, requiring group visits, offering cooking classes, guided exercise regimens, and behavior psychology visits, etc.); however the frequency of our MWM was more than most MWM arms in previous comparative studies looking at similar outcomes.21, 23
A recent study comparing MWM to surgery in morbidly obese patients (mean BMI 36) with more severe T2DM (mean HbA1c 9.2) revealed a 42% diabetes remission rate after sleeve gastrectomy.23 Interestingly, they found comparable hospitalization/readmission rates (9%) in the MWM group (including hospitalizations unrelated to MWM) and the sleeve group, likely due to the consequences of severe, poorly controlled diabetes. A different study comparing MWM to gastric banding in morbidly obese patients (mean BMI 37) with recently diagnosed T2DM (mean HbA1c 7.7) found a 73% remission rate.21 Our study, performed in a less obese population (mean BMI 32) with T2DM (mean HBA1c 7.8) revealed a 65% remission rate. Clinicians should consider earlier referral of obese patients with T2DM for bariatric surgery, as this may lead to a more significant impact on T2DM.
Conclusion
Bariatric surgery was very effective in the short-term in patients with T2DM and BMI 30–35. Surgery improved insulin resistance significantly compared to MWM and the short-term remission rate was 65%. Baseline sRAGE may predict patients most likely to benefit from surgery. These findings need to be confirmed with larger studies.
Acknowledgement
This project was supported by grant number K12HS019473 from the Agency for Healthcare Research and Quality. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Agency for Healthcare Research and Quality.
This project was also supported by the NYU-HHC Clinical and Translational Science Institute which is supported in part by grant 1UL1RR029893 from the National Center for Research Resources, National Institutes of Health
Footnotes
The authors declare there are no conflicts of interest.
Contributor Information
Manish Parikh, Department of Surgery, NYU School of Medicine/Bellevue Hospital.
Mimi Chung, Department of Surgery, NYU School of Medicine/Bellevue Hospital.
Sheetal Sheth, Department of Surgery, NYU School of Medicine/Bellevue Hospital.
Michelle McMacken, Department of Medicine, NYU School of Medicine/Bellevue Hospital.
Tasneem Zahra, Department of Medicine, Lincoln Hospital.
John K Saunders, Department of Surgery, NYU School of Medicine/Bellevue Hospital.
Aku Ude-Welcome, Department of Surgery, NYU School of Medicine/Bellevue Hospital Van Dunn MD MetroPlus Health Plan, Health and Hospitals Corporation.
Gbenga Ogedegbe, Department of Population Health, NYU School of Medicine/Bellevue Hospital.
Ann Marie Schmidt, Department of Medicine, NYU School of Medicine/Bellevue Hospital.
H Leon Pachter, Department of Surgery, NYU School of Medicine/Bellevue Hospital.
References
- 1.Buchwald H, Estok R, Fahrbach K, et al. Weight and type 2 diabetes after bariatric surgery: systematic review and meta-analysis. Am J Med. 2009;122:248–256. doi: 10.1016/j.amjmed.2008.09.041. [DOI] [PubMed] [Google Scholar]
- 2.Gastrointestinal Surgery for Severe Obesity National Institutes of Health Consensus Development Conference Statement March 25–27,1991. doi: 10.1093/ajcn/55.2.615s. http://consensus.nih.gov/1991/1991GISurgeryObesity084html.htm. [DOI] [PubMed]
- 3.Bays HE, Chapman RH, Grandy S, et al. The relationship of body mass index to diabetes mellitus, hypertension and dyslipidaemia: comparison of data from two national surveys. Int J Clin Pract. 2007;61:737–747. doi: 10.1111/j.1742-1241.2007.01336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maglione MA, Maggard Gibbons M, Livhits M, et al. Comparative Effectiveness Review No. 82. Rockville, MD: Agency for Healthcare Research and Quality; 2013. Jun, Bariatric surgery and nonsurgical therapy in adults with metabolic conditions and a body mass index of 30.0 to 34.9 kg/m2. (Prepared by the Southern California Evidence-based Practice Center under Contract No. 290-2007-10062-I.) AHRQ Publication No. 12(13)-EHC139-EF. [PubMed] [Google Scholar]
- 5.Cummings D. Metabolic surgery for type 2 diabetes. Nature Med. 2012;18:656–658. doi: 10.1038/nm.2773. [DOI] [PubMed] [Google Scholar]
- 6.Basta G, Lazerrini G, Massaro M, et al. Advanced glycation end products activate endothelium through signal-transduction receptor RAGE: a mechanism for amplification of inflammatory responses. Circulation. 2002;105:816–822. doi: 10.1161/hc0702.104183. [DOI] [PubMed] [Google Scholar]
- 7.Song F, Hurado El Pozo C, Rosario R, et al. RAGE regulates the metabolic and inflammatory response to high fat feeding in mice. Diabetes. 2014 Feb 11; doi: 10.2337/db13-1636. [epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brix J, Hollerl F, Kopp H, et al. The soluble form of the receptor of advanced glycation endproducts increases after bariatric surgery in morbid obesity. Inter J Obesity. 2012;36:1412–1417. doi: 10.1038/ijo.2012.107. [DOI] [PubMed] [Google Scholar]
- 9.Ramasamy R, Yan S, Schmidt A. RAGE: therapeutic target and biomarker of the inflammatory response – the evidence mounts. J Leukoc Biol. 2009;86:505–512. doi: 10.1189/jlb.0409230. [DOI] [PubMed] [Google Scholar]
- 10.Boutron I, Moher D, Altman DG, et al. Extending the CONSORT statement to randomized trials of nonpharmacologic treatment: explanation and elaboration. Ann Intern Med. 2008;148:295–309. doi: 10.7326/0003-4819-148-4-200802190-00008. [DOI] [PubMed] [Google Scholar]
- 11.Knowler W, Barrett-Connor E, Fowler S, et al. for the Diabetes Prevention Program. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pi-Sunyer X, Blackburn G, Brancati F, et al. Reduction in weight and cardiovascular disease risk factors in individuals with type 2 diabetes. Diabetes Care. 2007;30:1374–1383. doi: 10.2337/dc07-0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tuomilehto J, Lindström J, Eriksson J, et al. Prevention of type 2 diabetes by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344:1343–1350. doi: 10.1056/NEJM200105033441801. [DOI] [PubMed] [Google Scholar]
- 14.Wadden T, Berkowitz R, Womble L, et al. Randomized trial of lifestyle modification and pharmacotherapy for obesity. N Engl J Med. 2005;353:2111–2120. doi: 10.1056/NEJMoa050156. [DOI] [PubMed] [Google Scholar]
- 15.Parikh M, Dasari M, McMacken M, et al. Does a preoperative medically supervised weight loss program improve bariatric surgery outcomes? A pilot randomized study. Surg Endosc. 2012;26:853–861. doi: 10.1007/s00464-011-1966-9. [DOI] [PubMed] [Google Scholar]
- 16.Parikh M, Issa R, McCrillis A, et al. Surgical strategies that may decrease leak after laparoscopic sleeve gastrectomy; a systematic review and meta-analysis of 9991 cases. Ann Surg. 2013;257:231–237. doi: 10.1097/SLA.0b013e31826cc714. [DOI] [PubMed] [Google Scholar]
- 17.Aills L, Blankenship J, Buffington C, et al. ASMBS Allied Health Nutritional Guidelines for the Surgical Weight Loss Patient. Surg Obes Relat Dis. 2008;4:S73–S108. doi: 10.1016/j.soard.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 18.Shen R, Dugay G, Rajaram K, et al. Impact of patient follow-up on weight loss after bariatric surgery. Obes Surg. 2004;14:514–519. doi: 10.1381/096089204323013523. [DOI] [PubMed] [Google Scholar]
- 19.Selvin E, Steffes M, Zhu H, et al. Glycated hemoglobin, diabetes and cardiovascular risk in nondiabetic adults. N Engl J Med. 2010;362:800–811. doi: 10.1056/NEJMoa0908359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Robinson J, Lupkiewicz S, Palenik L, et al. Determination of ideal body weight for drug dosage calculations. Am J Hosp Pharm. 1983;40:1016–1019. [PubMed] [Google Scholar]
- 21.Dixon JB, O'Brien PE, Playfair J, et al. Adjustable gastric banding and conventional therapy for type 2 diabetes: a randomized controlled trial. JAMA. 2008;299:316–323. doi: 10.1001/jama.299.3.316. [DOI] [PubMed] [Google Scholar]
- 22.Parikh M, Issa R, Vieira D, et al. The role of bariatric surgery in the treatment of type 2 diabetes in patients who do not meet current NIH criteria (BMI< 35): A systematic review and meta-analysis. J Am Coll Surg. 2013;217:527–532. doi: 10.1016/j.jamcollsurg.2013.04.023. [DOI] [PubMed] [Google Scholar]
- 23.Schauer P, Kashyap S, Wolski K, et al. Bariatric surgery versus intensive medical therapy in obese patients with diabetes. New Engl J Med. 2012;366:1567–1576. doi: 10.1056/NEJMoa1200225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mingrone G, Panunzi S, De Gaetano A, et al. Bariatric surgery versus conventional medical therapy for type 2 diabetes. N Engl J Med. 2012;366:1577–1585. doi: 10.1056/NEJMoa1200111. [DOI] [PubMed] [Google Scholar]
- 25.Ikramuddin S, Korner J, Lee W, et al. Roux-en-Y gastric bypass vs. intensive medical management for the control of type 2 diabetes, hypertension and hyperlipidemia: the Diabetes Surgery Study randomized clinical trial. JAMA. 2013;309:2240–2249. doi: 10.1001/jama.2013.5835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.DePaula A, Stival A, Macedo A, et al. Prospective randomized controlled trial comparing 2 version of laparoscopic ileal interposition associated with sleeve gastrectomy for patients with type 2 diabetes with BMI 21–34. Surg Obes Relat Dis. 2010;6:296e305. doi: 10.1016/j.soard.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 27.Lee W, Chong K, Kong-Han S, et al. Gastric bypass vs. sleeve gastrectomy for type 2 diabetes mellitus. A randomized controlled trial. Arch Surg. 2011;146:143e148. doi: 10.1001/archsurg.2010.326. [DOI] [PubMed] [Google Scholar]
- 28.Arteburn D, Flum D, Westbrook E, et al. A population-based, shared decision-making approach to recruit for a randomized trial of bariatric surgery versus lifestyle for type 2 diabetes. Surg Obes Relat Dis. 2013;9:837–844. doi: 10.1016/j.soard.2013.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]