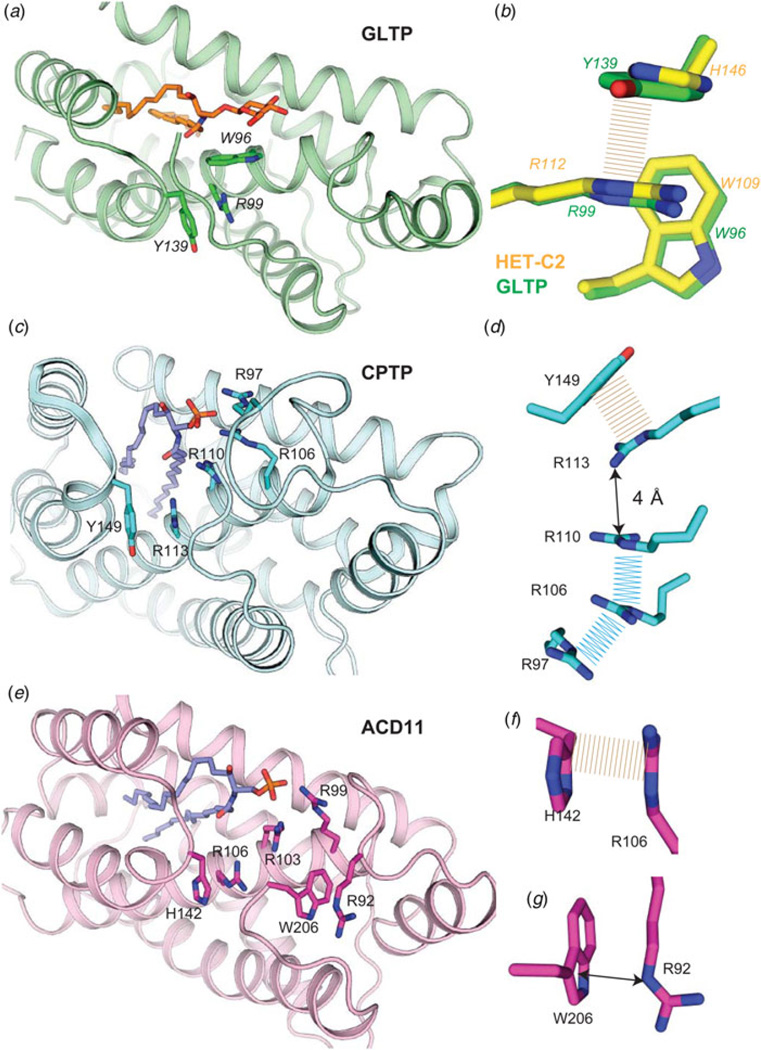
Fig. 8. Conserved cation–π and aromatic stacking (π–π) interactions that stabilize the sphingolipid headgroup recognition site in GLTP motifs.
(a) Human GLTP (green, ribbon representation) complexed with 18:1 GlcCer (orange stick representation). Trp96 (α4-helix) position is stabilized from beneath via cation-π interaction with Arg99 (α4-helix) which maintains orientation by π-π stacking with Tyr139 (α5–α6 loop). (b) Zoomed enlargement of side-chain interactions in a) (viewed from beneath) and superposition with residues (Trp109, Arg112, His146; yellow stick representation) at equivalent positions in the fungal GLTP homolog, HET-C2. (c) Human CPTP (cyan, ribbon representation) complexed with 16:0-C1P (blue stick representation). Arg110 (α4-helix) position is stabilized via π–π stacking (or perhaps cation–π interaction) with Arg113 (α4-helix) which maintains orientation by π-π stacking with Tyr149 (α5-α6 loop). (d ) Zoomed enlargement of side-chain interactions in c) (viewed from beneath) including additional π-π stacking interactions involving Arg110, Arg106, and Arg97 and located nearby. (e) Arabidopsis ACD11 (cameo, ribbon representation) complexed with 16:0-C1P (blue stick representation) by modeling. Arg103 (α4-helix) positioning might not be stabilized by cation–π interaction with Arg106 (α4-helix) which maintains orientation by π–π stacking with His142 (α5–α6 loop). (f) Zoomed enlargement of side-chain interactions involving π–π stacking between Arg106 (α4-helix) and His142 (α5–α6 loop) in e) (viewed from beneath). (g) ACD11 positioning of C-terminus (Trp206) appears to be stabilized by cation–π interaction with Arg92.