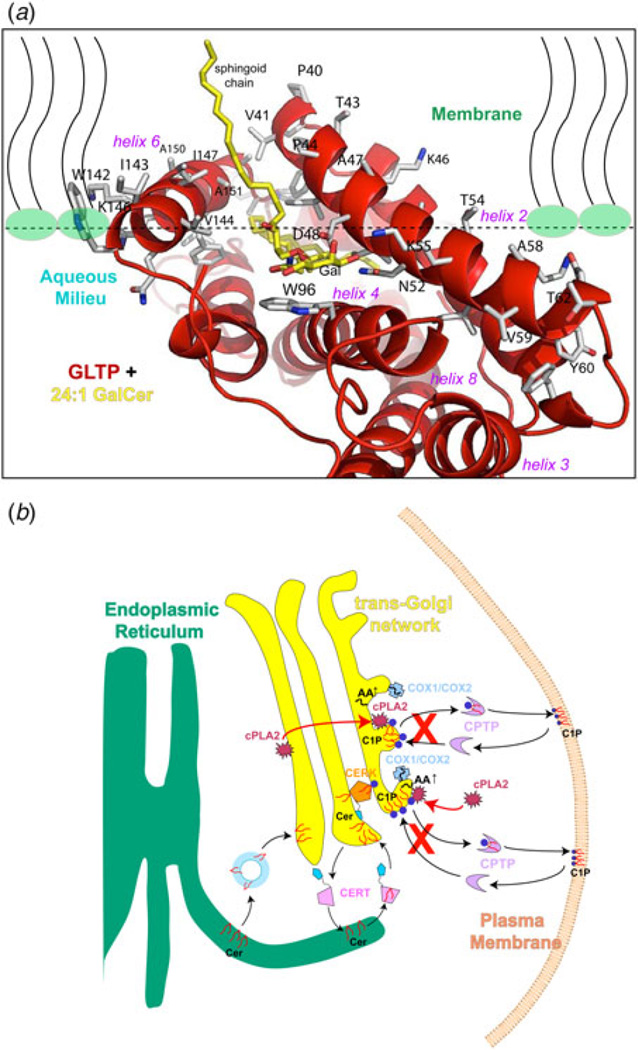
Fig. 9. Models for GLTP interaction with membranes and for regulation of pro-inflammatory eicosanoid production by CPTP.
(a) Model for GLTP orientation and positioning with the membrane. Residues involved in initial docking of GLTP with the membrane interface were identified using the orientation of proteins in membranes (OPM) computational approach (Lomize et al. 2011). Lipid molecules, comprising half of the membrane, are shown with wavy lines (black) to represent the lipid hydrocarbon chains and elliptical headgroups (green-fill). Also shown are GLTP helices (red ribbons), Trp (cyan), and important other side-chain residues (black) for membrane interaction, and 24:1-GalCer (yellow-stick; sphingosine-out mode). In this mode, the sphingoid chain is shown exiting the PC matrix, while the 24:1 acyl chain extends (away from view) roughly parallel with the protein–membrane interface, before assuming a serpentine conformation deep in the hydrophobic pocket (see Fig. 5a for another view). The membrane docking positions for HET-C2, FAPP2–GLTPH, CPTP, and ACD11 are expected to be similar (Kamlekar et al. 2013; Kenoth et al. 2010; Simanshu et al. 2013, 2014). (b) Model for CPTP intracellular regulation of eicosanoid production. C1P is normally synthesized by CERK which can concentrate in the trans-Golgi network vicinity via its PH domain. To produce C1P, CERK uses Cer transported from the ER by CERT that also contains a targeting PH domain. After synthesis in the trans-Golgi, C1P is transported to subcellular destinations by CPTP and possibly by vesicular trafficking. RNAi knockdown of CPTP (shown by red Xs) leads to accumulation and elevation of C1P at the Golgi complex, a condition that activates soluble cytosolic phospholipase A2α (cPLA2α), and generates arachidonic acid needed for downstream elevations of pro-inflammatory eicosanoids. CPTP overexpression has the opposite effect on lipid levels including suppression of pro-inflammatory eicosanoid production (see Supplementary Figures S12 and S14 in Simanshu et al. 2013). cPLA2α activation occurs by translocation from the cytoplasm and/or cis-Golgi (red arrows) and enhanced anchoring at the TGN via CERK-generated C1P. COX-1 and inducible COX-2 which use arachidonic acid to produce pro-inflammatory prostaglandins, also concentrate in the TGN vicinity during stimulation. For clarity, other pathways of eicosanoid generation, that is, LOX (cytoplasmic 5-lipoxygenase) and CYP (ER-associated cytochrome P450), are not depicted. Also not depicted is Golgi cisternal stack fragmentation induced by CPTP RNAi for 24 h.