Abstract
The DC-SIGN receptor on human dendritic cells interacts with HIV gp120 to promote both infection of antigen-presenting cells and transinfection of T cells. We hypothesized that in DC-SIGN-expressing cells, both DC-SIGN ligands such as dextrans and gp120 antagonists such as peptide triazoles would inhibit HIV infection with potential complementary antagonist effects. To test this hypothesis, we evaluated the effects of dextran (D66), isomaltooligosaccharides (D06), and several peptide triazoles (HNG156, K13, and UM15) on HIV infection of B-THP-1/DC-SIGN cells. In surface plasmon resonance competition assays, D66 (IC50 = 35.4 μM) and D06 (IC50 = 3.4 mM) prevented binding of soluble DC-SIGN to immobilized mannosylated bovine serum albumin (BSA). An efficacious dose-dependent inhibition of DC-SIGN-mediated HIV infection in both pretreatment and posttreatment settings was observed, as indicated by inhibitory potentials (EC50) [D66 (8 μM), D06 (48 mM), HNG156 (40 μM), UM15 (100 nM), and K13 (25 nM)]. Importantly, both dextrans and peptide triazoles significantly decreased HIV gag RNA levels [D66 (7-fold), D06 (13-fold), HNG156 (7-fold), K-13 (3-fold), and UM15 (6-fold)]. Interestingly, D06 at the highest effective concentration showed a 14-fold decrease of infection, while its combination with 50 μM HNG156 showed a 26-fold decrease. Hence, these compounds can combine to inactivate the viruses and suppress DC-SIGN-mediated virus–cell interaction that as shown earlier leads to dendritic cell HIV infection and transinfection dependent on the DC-SIGN receptor.
Human immunodeficiency virus (HIV) infection leads to the death of over 1.5 million people every year, more than any other infection.1 Dendritic cells (DCs) are known participants in the pathogenesis of both active and latent HIV infection.2,3 The DC surface receptor, DC-SIGN (dendritic cell-specific ICAM-3 grabbing nonintegrin), binds HIV envelope glycoprotein gp120 and facilitates transinfection of the main HIV targets––the CD4+ T cells.4,5 HIV transinfection of T cells by DCs is not dependent exclusively on DC-SIGN and although the main molecule driving transinfection is considered to be Siglec-1/CD169,6,7 DC-SIGN remains an important HIV-binding receptor.
The role of DC-SIGN in HIV infection in vivo is not fully understood. The decreased DC-SIGN expression in HIV may be associated with reduced mother-to-child transmission,8 and some DC-SIGN genetic variants that likely reduce its functionality are more prevalent in the HIV-negative population.9 Therefore inhibiting viral entry via DC-SIGN is a promising approach for complex HIV therapy.10,11 Virus entry inhibitors that interact with viral protein gp41 and HIV cellular receptor CCR5 are already utilized in clinical practice, while inhibitors of other HIV receptors such as CD4 and CXCR4 have been or are currently being tested in clinical trials.12 Inhibitors of HIV infection and transinfection via the DC-SIGN lectin receptor have been tested only in preclinical studies.13–15 Development or application of known drugs for the inhibition of HIV/DC-SIGN interaction may help in decreasing transinfection of T cells and in preventing the infection of DCs that serve as a long-term reservoir of HIV.2
Utilizing different glycan or glycosylated structures is one experimental approach to inhibit the lectin receptors. Dextrans and oligodextrans are DC-SIGN ligands. It is known that dextran uptake in the DCs is dependent on DC-SIGN and mannose receptor expression.16 However, it has not been reported if dextrans could be utilized as effective DC-SIGN inhibitors. In addition, it has not been demonstrated that oligosaccharides with the linear dextran structure (α-1,6-linked glucose units) bind DC-SIGN.17 Hence, we proposed to utilize the dextran molecule, as it is part of several injectable drug formulations that have a proven safety record, as an inhibitor of pathogen interaction with a set of C-type lectins including DC-SIGN (CD209) and mannose receptor (CD206).17 In addition, we tested whether peptide triazoles could inhibit DC-SIGN-mediated HIV infection. These compounds have proven to be effective inhibitors of HIV infection mediated by CD4 and coreceptors CCR5/CXCR418 due to gp120 shedding and, in the case of test compound KR13, by additional lytic release of the viral capsid protein p24.19 Finally, we tested the potential for complementarity between peptide triazoles and dextrans in inhibiting DC-SIGN-mediated HIV infection.
We first investigated the binding properties of D66 and D06 toward DC-SIGN using a surface plasmon resonance (SPR) competition assay. DC-SIGN extracellular domain (DC-SIGN ECD) construct was produced and purified as described previously.20 Dextrans of average molecular mass 66,000 Da (D66) (MP Biomedicals, USA) or Vita Fiber (D06) (BioNeutra, Canada) were utilized for these studies. This source of dextran oligomers is a mixture of glucose (15.5%) and isomaltooligosaccharides (isomaltose, isomaltotriose, and longer molecules containing up to seven glucose units) with an average molecular mass of 560 Da. SPR competition assays were performed on a Biacore 3000 using a CM4 chip, functionalized at 5 μl/min.
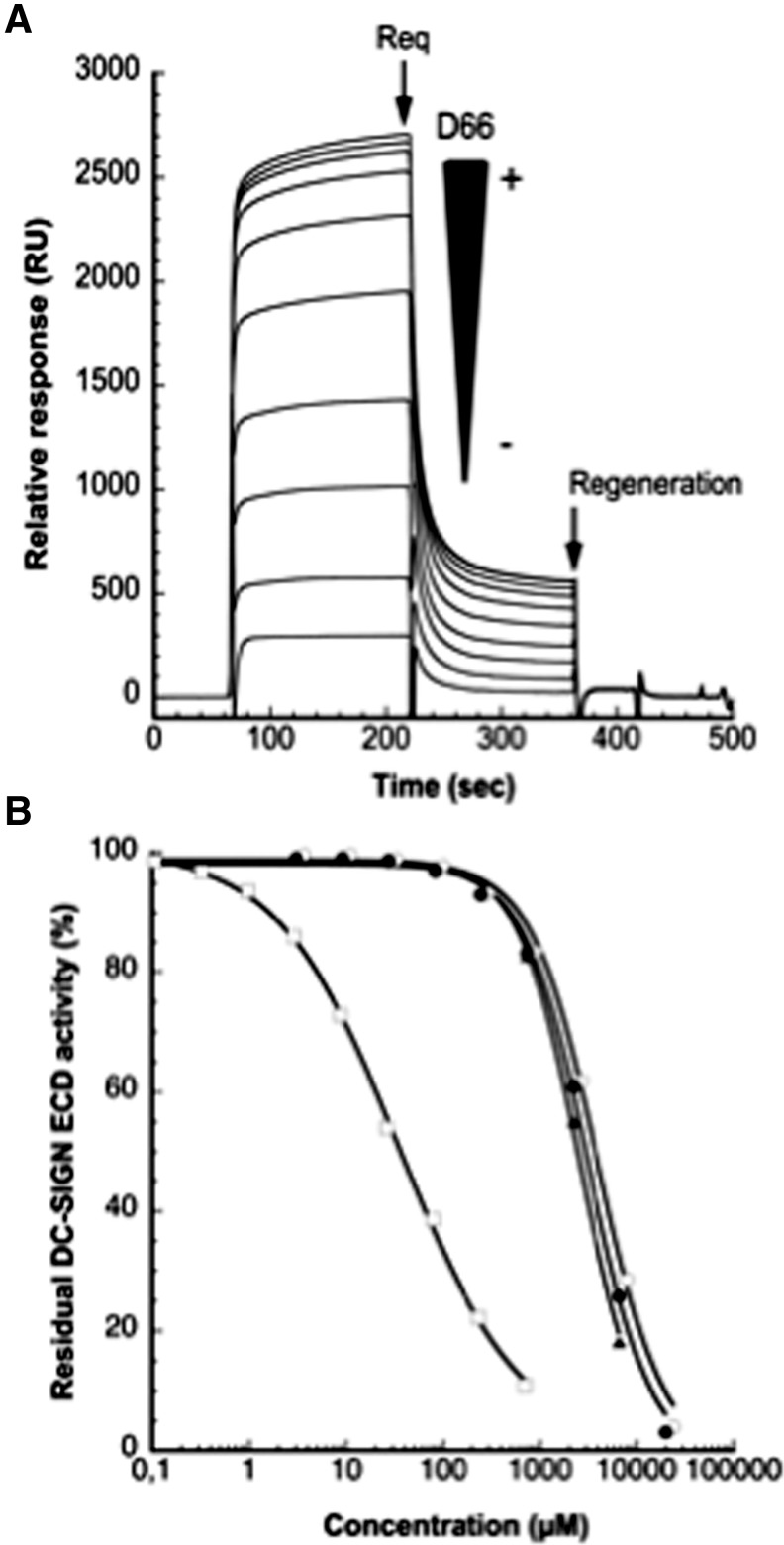
Bovine serum albumin (BSA) or mannosylated bovine serum albumin (BSA-Man) was immobilized on flow cells by amine coupling. The commercially available BSA-Man utilized in these assays contains an average of 12 glycosylations sites displaying the Man α-1,3 [Man α-1,6] Man branched trisaccharide. BSA-Man was covalently attached to a CM4 sensor chip. For inhibition studies, 20 μM DC-SIGN ECD was mixed with increasing concentrations of the inhibiting compounds. The percentage inhibition (IC50) of DC-SIGN binding to immobilized BSA-Man was calculated from the resulting sensorgrams that were reference surface corrected. Inhibition studies were performed using DC-SIGN ECD (20 μM) introduced alone or in the presence of increasing concentrations of D66 or D06. D66 (Fig. 1A) and D06 (sensorgrams not shown) dose-dependently inhibited binding between DC-SIGN and BSA-Man. Based on the inhibition curve shown in Fig. 1B, we determined the IC50 values for D66 and D06. The affinity of the short oligodextran molecule (isomaltooligosaccharide) D06 (IC50 = 3.4 mM) for the DC-SIGN receptor was comparable to that of monovalent d-mannose (IC50 = 3.1 mM) and fucose (IC50 = 2.5 mM) (Fig. 1B). This suggests that the D06 binding to the DC-SIGN receptor could occur by interaction with one glucose unit at a time.
FIG. 1.
Inhibition of DC-SIGN extracellular domain (DC-SIGN ECD) binding to immobilized mannosylated-bovine serum albumin (BSA) using surface plasmon resonance (SPR). (A) DC-SIGN ECD (20 μM) was coinjected with D66 at an increasing concentration from 0 to 704 μM onto the mannosylated-BSA functionalized BIA 3000 chip surface (1847 RU). Black arrow Req represents the maximal binding response at equilibrium. The regeneration arrow corresponds to the EDTA injection for surface regeneration between each cycle. (B) Comparison of the inhibitory potency of l-fucose (▴), D66 (□), D06 (○), and d-mannose (●) toward the DC-SIGN ECD mannosylated-BSA interaction determined by competition assays. IC50 values were inserted in Table 1.
D66 binding to DC-SIGN was much more potent compared to D06. Inhibition of the DC-SIGN/BSA-Man interaction by 50% using D66 could be achieved with an approximately 100 times lower dose (IC50 = 35.4 μM) as compared to D06 (Table 1). Of note, there are approximately 100 times more glucose units in D66 than in D06. Hence, while the inhibitory potencies (IC50 w/v) of D66 and D06 are comparable, a comparison on a molar concentration basis results in a strikingly different potency (IC50 μM, Table 1). Hence, a linear presentation of glucose in a high-molecular-weight dextran (D66) does not potentiate DC-SIGN binding of all of the glucose units, since the mass quantites required for 50% inhibition from D06 and D66 were similar. Any glucose in the linear dextran molecule is similarly accessible for interaction in both D66 and D06. Indeed, if only terminal units were able to interact within the active site of the DC-SIGN receptor, a much higher IC50 w/v value would be anticipated for D66 as compared to D06. Thus, the α-1,6-linkage in D66 does not induce steric hindrance of one neighboring glucose unit to the other within the linear chain of dextran, and for any individual glucose unit the hydroxyls in position 3 and 4 appear equally competent to coordinate the Ca2+ in the DC-SIGN binding site.
Table 1.
IC50 Expressed in Molar Concentration, Mass Concentration, or Percent Weight/Volume
| IC50 ± SD(μM) | IC50(mg/ml) | IC50 w/v(%) | |
|---|---|---|---|
| l-Fucose | 2480.0 ± 17.3 | 0.40 | 0.04 |
| d-Mannose | 3057.0 ± 66.0 | 0.55 | 0.05 |
| D06 | 4070.0 ± 70.0 | 2.03 | 0.20 |
| D66 | 35.4 ± 1.6 | 2.33 | 0.23 |
We next investigated whether dextrans inhibit HIV infection of B-THP-1/DC-SIGN cells. Human B cell lines B-THP-1 and B-THP-1/DC-SIGN were infected with pseudotyped HIV-1 and treated with D66 or D06. Pseudovirus for a single round of infection was obtained from HEK 293T cells with pNL4-3.LUC.R-E- backbone plasmid and BaL.01 env-expressing plasmid as previously described.21 Cells were seeded in 12-well plates with 1 million cells per well in 1 ml of growth medium and were infected with 3 ng p24gag of virus. Test compounds were added 30 min before infection (preinfection treatment) or 20 h after infection (postinfection treatment). Twenty-four hours after infection, cells were thoroughly washed (to analyze intracellular and surface-bound virus) or trypsinized and washed (to analyze only intracellular virus, default conditions), after which the total RNA was isolated. TRI Reagent (Life Technologies, USA) was utilized for RNA isolation. QuantiTect kit (Qiagen, USA) was utilized for cDNA synthesis. cDNA amplification of the HIV gag gene was performed with a pair of forward Gag-F (5′-CTA GAA CGA TTC GCA GTT AAT CCT-3′) and reverse Gag-R (5′-CTA TCC TTT GAT GCA CAC AAT AGA G-3′) primers.22 GAPDH was utilized as a housekeeping control gene.
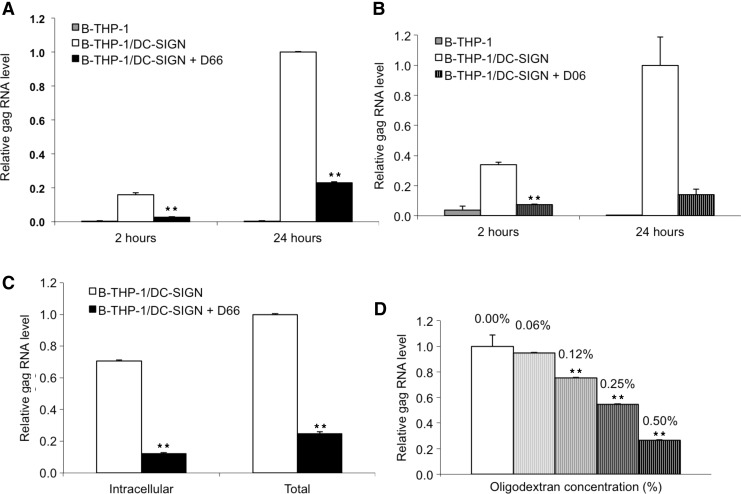
B-THP-1 cells were infected with HIV only when they expressed the DC-SIGN receptor (Fig. 2A). Both D66 and D06 efficaciously inhibited infection of B-THP-1/DC-SIGN cells with HIV. At 24-h postinfection, D66 and D06 decreased RNA gag levels to 23% and 14% of control infection, respectively (Fig. 2A and B). B-THP cells did not have any detectable gag RNA transcripts. Hence, the decrease of HIV infection in DC-SIGN-expressing cells was likely due to the inhibition of HIV-DC-SIGN interactions. A possible concern about HIV replication in our model may occur. Indeed, 2 h is enough for HIV endocytosis to reach saturation in permissive cell lines,12 while 24 h is time enough for replication.13 And in our study the 5-fold increase of HIV RNA associated with cells from 2 h to 24 h might seem a sign of replication. But virus in the infected cells presumably have not replicated because (1) the B-THP-1/DC-SIGN cell line is almost identical to the Raji/DC-SIGN14 and (2) endocytosis of HIV in Raji/DC-SIGN cells is not observed and they are not permissive to HIV infection.15 While the fact that Raji/DC-SIGN cells are not permissive for HIV is well proven,15 another work more convincingly shows most of HIV gp120 is internalized in Raji/DC-SIGN cells while a smaller share stays bound on the cell surface and the ratio conforms to our data.16 The increase of virus-per-cell number between 2 and 24 h may be explained mainly by upregulation of DC-SIGN expression by HIV,17 but not its replication.
FIG. 2.
Dextran and oligodextran inhibition of DC-SIGN-dependent HIV infection. (A, B) Dextran and oligodextran decreased HIV infection of the B-THP-1/DC-SIGN cells. Cells were incubated for 30 min in a 0.5% solution (760 μM) of dextran with average molecular mass 66,000 or oligodextran, and then infected with pseudotyped HIV virus. After 24 h, cells were harvested and viral RNA contents were measured by polymerase chain reaction (PCR). Dextran-treated cells contained a four times lower amount of viral RNA than control cells. Cells that do not express DC-SIGN (B-THP-1) were not infected under the same conditions. (C) Intracellular and total HIV RNA gag levels decreased in B-THP-1/DC-SIGN cells in response to treatment with dextran, even when applied postinfection. Cells were incubated with pseudotyped HIV virus for 20 h and then treated with 0.5% dextran. After 4 h, cells were harvested. The addition of dextran decreased infection of the cells four times. (D) Oligodextran dose dependently decreased HIV infection of the B-THP-1/DC-SIGN cells. Cells incubated for 30 min in the serial dilutions of oligodextran, starting from 0.5% (100 mM), were infected with pseudotyped HIV virus. After 24 h, cells were harvested and the viral RNA contents were measured by PCR. Results are presented as mean ± SD. Statistical differences were estimated using Student's t-test for pair comparisons and are shown as **p < 0.001.
D66 decreased DC-SIGN-dependent HIV infection even when B-THP-1/DC-SIGN cells were treated at 20 h postinfection. When cells were treated with D66, the amount of intracellular and total HIV gag RNA after infection dropped to 17% and 25% of control, respectively (Fig. 2C). In fact, if we take into account the decreases in intracellular HIV gag RNA as well as similar decreases on the cell surface, it may be inferred that D66 substantially decreases the amount of cell surface-associated viral RNA. The dextran solution utilized in this study is analogous to the clinical dextrans, which are blood plasma substitutes. Usually they are inexpensive drugs with outstanding biocompatibility that are able to circulate at high concentrations into the bloodstream for several days after injections.23
D06 inhibited DC-SIGN-dependent HIV infection of B-THP-1/DC-SIGN cells in a dose-dependent manner. At a range of concentrations, the level of HIV gag RNA was inversely related to D06 concentration (EC50 = 48 mM) (Fig. 2D). It is still not clear why higher concentrations do not inhibit infection more strongly for both D66 and D06 (data not shown). Possible explanations include limited access of the virus–cell interface, for example, due to multivalent attachment of virus and cell.
The exact mechanism for dextrans to inhibit DC-SIGN-dependent HIV infection in both preinfection and postinfection treatment settings is not known. The high-molecular-mass dextrans, known as ligands of DC-SIGN,16 are able to activate endocytosis and to be transferred into cells. It will be interesting to evaluate the interference of their internalization properties with their potential competition with virus for DC-SIGN binding. Smaller dextrans are not active inductors of endocytosis,24 while even oligodextrans contain enough glucose residues (3–4) to bind the active site of the DC-SIGN carbohydrate recognition domain.25 We surmise that there is a balance of endocytosis and exocytosis processes that leads to a permanent exchange of the membrane between intracellular vesicles and plasmalemma. Viral particles bound to the receptor, and being already endocytosed inside the cell, are able to come out to the cell surface. Dextrans possibly compete with virus on the cell surface for binding to DC-SIGN. When the membrane goes back inside the cell, it carries receptor-bound dextran instead of virus.
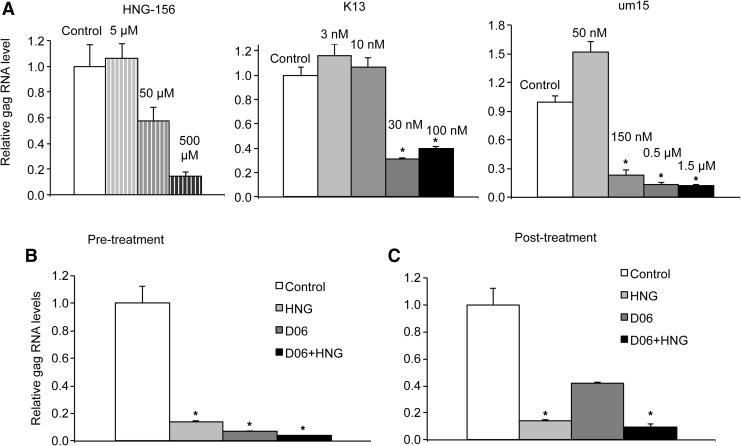
We next investigated whether peptide triazoles could inhibit HIV infection of B-THP-1/DC-SIGN cells. The structures, gp120 binding potencies, and antiviral activities of the peptide triazoles HNG-156, KR13, and UM15 utilized in this study have been described previously.5,19,26 All of these compounds (added to the viral suspensions 30 min before infection) were found to exert a dose-dependent inhibitory effect on the HIV infection of B-THP-1/DC-SIGN cells. At 24 h postinfection there was a 7-fold, 3-fold, and 6-fold decrease in HIV gag RNA with the highest concentrations of HNG-156, K13, and UM15, respectively (Fig. 3A). This inhibition of HIV infection was efficacious and dose dependent for each of the peptide triazoles that we tested: HNG-156 (EC50 = 40 μM), K13 (EC50 = 25 nM), and UM15 (EC50 = 100 nM).
FIG. 3.
Peptide triazoles inhibit DC-SIGN-dependent HIV infection. (A) HNG-156, K13, and UM15 show dose-dependent inhibition of the pseudotyped HIV infection of B-THP-1/DC-SIGN cells. Intact virus or virus pretreated with compounds for 30 min was added to the cells, and the rate of viral RNA associated with cells was measured 24 h postinfection. (B) Both 50 μM HNG-156 (HNG) and 100 mM oligodextran (D06) show complementarity in preventing HIV infection of B-THP-1/DC-SIGN cells. (C) In the posttreatment analysis, both were added 20 h postinfection and still decreased infection. Results are given as mean ± SD. The significance of the statistical differences is calculated by Student's t-test and is shown as *p < 0.01.
Finally, we investigated the combined activities of peptide triazoles and dextrans. We tested inhibition of HIV infection in both preinfection or postinfection treatment settings, and determined if these compounds acted complementarily. HNG-156 and D06 demonstrated stronger inhibition in DC-SIGN-dependent HIV infection of B-THP-1/DC-SIGN cells when applied simultaneously in both preinfection and postinfection treatment settings (Fig. 3B and C). In the preinfection treatment setting, 50 μM HNG-156 resulted in a 7-fold decrease in HIV gag RNA, and 100 mM (0.5%) D06 resulted in a 14-fold decrease as compared to control, respectively (Fig. 3B). When viral particles/cells were pretreated with HNG-156 and D06 respectively, HIV gag RNA decreased by 26-fold vs. control in 24 h (Fig. 3B). In the D06 postinfection treatment setting, 100 mM (0.5%) D06 resulted in a 2.4-fold decrease as compared to control (Fig. 3B). When cells/virus were treated with both of these compounds, HIV gag RNA decreased by 10-fold vs. control in 24 h (Fig. 3C).
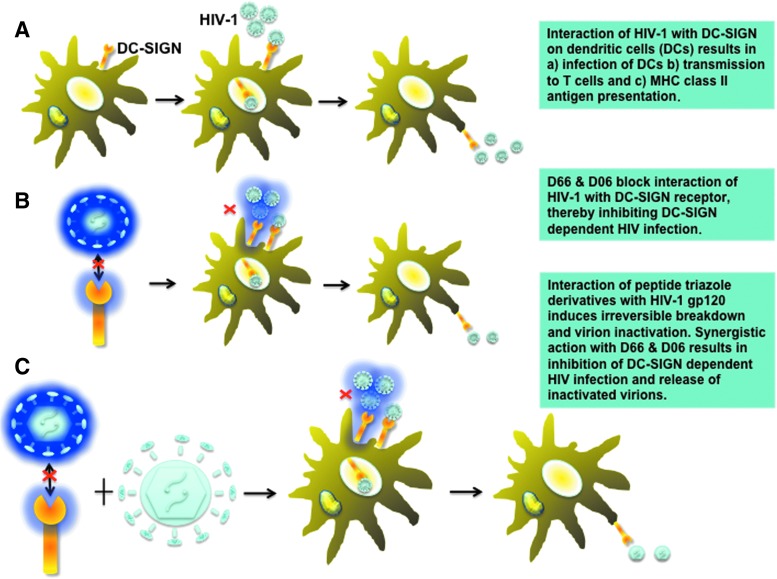
The mechanisms through which dextrans and peptide triazoles affect cell infection with HIV gp120 are significantly different. Dextrans bind DC-SIGN and directly inhibit HIV-cell interactions via this receptor. In contrast, peptide triazole binding to Env gp120 causes virus inactivation independent of cell effects and hence will inactivate virus either before the cell encounter or when virus is released from the cells. These differences are highlighted in Fig. 4.
FIG. 4.
Mechanisms through which dextrans and peptide triazoles inhibit DC-SIGN-mediated HIV infection. (A) Interaction of HIV-1 with DC-SIGN expressed on dendritic cells (DCs) results in (a) infection of DCs and (b) transmission of the virus to CD4+ T cells and antigen presentation through MHC class II molecules. (B) Dextrans block HIV-DC-SIGN interaction resulting in a decreased infection. The quantity of HIV-DC-SIGN complex cycling through the endosome pool is decreased. (C) Interaction of peptide triazoles with HIV-1 gp120 induces irreversible breakdown and virion activation. Dextran and peptide triazole act complementarily, decreasing the amount of virus through irreversible inactivation combined with decreasing the quantity of HIV-DC-SIGN complex cycling through the endosome pool. Color images available online at www.liebertpub.com/aid
Detrimental side effects of the studied compounds on cellular functions are of low probability. Dextran has been used at a concentration of 32% by mass intraperitoneally in clinical settings,27 it shows no cytotoxic effects in human fibroblasts at concentrations at least up to 4% by mass,28 it shows no cytotoxicity in macrophages, and also does not influence the production of interferon (IFN)-γ and interleukin (IL)-10 at a concentration of 0.5% by mass (S. Pustylnikov, unpublished data). Oligodextran also shows low or no systemic toxicity when given orally as a 3% solution29 and no cytotoxicity in macrophages at a concentration of 0.5% by mass (S. Pustylnikov, unpublished data). Therefore high EC50 values for these compounds are balanced with their low toxicity. Peptide triazoles are also not toxic to cells at the studied concentrations.19,26
There is an urgent need to develop new effective and affordable strategies against HIV, taking into account the chronic character of this disease (in case of permanent treatment) that necessitates the use of drugs with the lowest side effects possible.30 To the best of our knowledge, this is the first study to apply dextran/oligodextran as a base for the development of biocompatible DC-SIGN and MR (CD206) ligands in the setting of infections including HIV and tuberculosis. HIV therapy requires the lifelong administration of drugs and this limits the application of intravenous drugs such as high-molecular-weight dextran. However, dextrans may inhibit DC-SIGN-dependent HIV infection when utilized in gel formulations (at concentrations many fold higher than used in this study) for topical applications to prevent HIV sexual transmission.
An advantage of oligodextrans (compared to polymeric dextrans) is the higher potential for the development of oral medications. Though gut adsorption of the dextran with an average molecular mass of 4000 (on average six to seven times larger molecules than oligodextran used in this study) does not lead to an increase in blood plasma concentration,31 improving the drug's oral bioavailability is a solvable task.32 Currently, isomaltooligosaccharides are used as a popular food supplement (prebiotic, fiber).33 Our observations regarding their DC-SIGN binding properties may expand the sphere of their application, but also raise some questions about their potential influence on the immune responses when they are utilized as food supplements. Possibly we are increasing our understanding of the microbiota-independent, lectin-dependent mechanisms of their immunomodulatory effects.34
We identified currently used drug dextran and food supplement isomaltooligosaccharides as DC-SIGN inhibitors and now they may find new applications in therapy for numerous infections17 including HIV. Several-fold infection inhibition provided by dextrans (and many other DC-SIGN inhibitors that bind the carbohydrate recognition domain) might be therapeutically challenging. However, the efficiency of binding could be enhanced by chemical modifications. Nonetheless, dextrans may be utilized safely in high doses and use of oligodextrans as food supplements suggests that they are very safe.
DC-SIGN is an important receptor of immune cells (primarily DCs) that helps pathogens to invade the cells both cis and trans, and to modulate an immune response, in many cases helping pathogens to survive.35 The relevance of B cells with a high level of DC-SIGN expression as a model for DCs needs to be further elucidated. However, the mechanisms of initial DC-SIGN-HIV interaction and endocytosis are likely to be the same in B cells and DCs. Moreover, it is known that activated B cells express DC-SIGN36 and HIV infection of such cells would also be prevented as shown in our study. DC-SIGN inhibitors are of special interest as a means of preventing DC infection and CD4+ T cell transinfection by HIV. Many potential DC-SIGN inhibitors are in various stages of research,13–15 and approaches for the high-throughput screening of potential inhibitors are being developed.37
Our earlier studies on human macrophages38 demonstrate that dextrans are also inhibitors of the interaction of HIV with another C-type lectin MR (CD206). This latter receptor binds HIV on the surface of macrophages, also allowing them to transinfect the T cells,39 and mediates the HIV infection of brain macrophages.40 Binding of HIV via MR increases the production of matrix metalloproteinase 2 by astrocytes in the brain.41 Therefore, MR is probably important for the creation of latent infection reservoirs in the brain.
In the current study, by utilizing surface plasmon resonance we demonstrated that high-molecular-weight dextran and smaller dextran oligomers, also known as oligodextrans or isomaltooligosaccharides, are specific DC-SIGN ligands. Importantly, these compounds efficaciously inhibited DC-SIGN-dependent HIV infection of B-THP-1/DC-SIGN cells in a dose-dependent manner in both pretreatment and posttreatment settings. Peptide triazoles also efficaciously inhibited DC-SIGN-dependent HIV infection in a similar manner. The results suggest a new and potentially useful activity for this class of anti-HIV compounds. These molecules were known to block virus cell entry, to induce shedding of HIV envelope protein gp120, and for peptide triazole thiols to cause membrane disruption leading to capsid protein leakage,18,19 all of which promote inhibition of gp120-dependent infection of the T cells. In our model we have shown that these compounds complement the inhibition of the gp120-dependent binding of HIV particles to DC-SIGN by dextrans.
Both dextrans and peptide triazoles decreased the level of HIV RNA associated with cells when applied 20 h postinfection. In case of dextran this is surmised to be due to the (1) balance between endocytosis and exocytosis: the exchange of membrane between plasmalemma and intracellular endosomal compartment and (2) equilibrium between virus and dextran bound to the cell surface. Process (1) leads to the transition of intracellular virus to the cell surface, while process (2) provides exchange of receptor-associated virus for dextran. In the case of peptide triazoles process (1) exposes virus on the cell surface where it becomes available for interaction with peptide triazoles. Simultaneous use of two strategies (peptide triazoles and dextrans) allowed for a stronger decrease of HIV infection compared to the maximum inhibition reached using either of the strategies alone.
In the future, dextrans could be tested in other models of infection in which the pathogens enter the cells, to induce immunomodulatory signaling, via a set of dextran-binding receptors, including DC-SIGN, L-SIGN, MR, and langerin.17 Such pathogens induce deadly infections (Ebola virus, tuberculosis, HIV/AIDS, influenza) and affect the nervous system (tick-borne encephalitis). Both dextrans and peptide triazoles can potentially be used in the prevention of DC-SIGN-dependent HIV infection.
Acknowledgments
This work was supported by the Novosibirsk Tuberculosis Research Institute. P.J. and Z.K.K. were supported by the U.S. Public Health Service/National Institutes of Health Grants AI077414 and R56AI077414. A.A.R. and I.C. were supported in this work by NIH P01 GM56550. V.P. was supported by a grant from la Région Rhône-Alpes. Authors also acknowledge Ms. Rashida Ginwala's efforts in proofreading the manuscript.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Ford N, Vitoria M, Hirnschall G, and Doherty M: Getting to zero HIV deaths: Progress, challenges and ways forward. J Int AIDS Soc 2013;16(1):18927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coleman CM. and Wu L: HIV interactions with monocytes and dendritic cells: Viral latency and reservoirs. Retrovirology 2009;6(1):51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahmed Z, Kawamura T, Shimada S, and Piguet V: The role of human dendritic cells in HIV-1 infection. J Invest Dermatol 2015;135(5):1225–1233 [DOI] [PubMed] [Google Scholar]
- 4.Hodges A, Sharrocks K, Edelmann M, et al. : Activation of the lectin DC-SIGN induces an immature dendritic cell phenotype triggering Rho-GTPase activity required for HIV-1 replication. Nat Immunol 2007;8(6):569–577 [DOI] [PubMed] [Google Scholar]
- 5.Coleman CM, Gelais CS, and Wu L: Cellular and viral mechanisms of HIV-1 transmission mediated by dendritic cells. Adv Exp Med Biol 2013;762:109–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Izquierdo-Useros N, Lorizate M, McLaren PJ, et al. : HIV-1 capture and transmission by dendritic cells: The role of viral glycolipids and the cellular receptor Siglec-1. PLoS Pathog 2014:10(7): e1004146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kijewski SDG. and Gummuluru S: A mechanistic overview of dendritic cell-mediated HIV-1 trans infection: The story so far. Future Virol 2015;10(3):257–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boily-Larouche G, Milev MP, Zijenah LS, et al. : Naturally-occurring genetic variants in human DC-SIGN increase HIV-1 capture, cell-transfer and risk of mother-to-child transmission. PloS One 2012;7(7):e40706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang J, Zhang X, Fu J, et al. : Protective role of DC-SIGN (CD209) neck-region alleles with <5 repeat units in HIV-1 transmission. J Infect Dis 2008;198(1):68–71 [DOI] [PubMed] [Google Scholar]
- 10.Qian K, Morris-Natschke SL, and Lee KH: HIV entry inhibitors and their potential in HIV therapy. Med Res Rev 2009;29(2):369–393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Flores A. and Quesada E: Entry inhibitors directed towards glycoprotein gp120: An overview on a promising target for HIV-1 therapy. Curr Med Chem 2013;20(6):751–771 [PubMed] [Google Scholar]
- 12.Henrich TJ. and Kuritzkes DR: HIV-1 entry inhibitors: Recent development and clinical use. Curr Opin Virol 2013;3(1):51–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alexandre KB, Gray ES, Mufhandu H, et al. : The lectins griffithsin, cyanovirin-N and scytovirin inhibit HIV-1 binding to the DC-SIGN receptor and transfer to CD4+ cells. Virology 2012;423(2):175–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Varga N, Sutkeviciute I, Ribeiro-Viana R, et al. : A multivalent inhibitor of the DC-SIGN dependent uptake of HIV-1 and dengue virus. Biomaterials 2014;35(13):4175–4184 [DOI] [PubMed] [Google Scholar]
- 15.Thépaut M, Guzzi C, Sutkeviciute I, et al. : Structure of a glycomimetic ligand in the carbohydrate recognition domain of C-type lectin DC-SIGN. Structural requirements for selectivity and ligand design. J Am Chem Soc 2013;135(7):2518–2529 [DOI] [PubMed] [Google Scholar]
- 16.Garcia-Nieto S, Johal RK, Shakesheff KM, et al. : Laminin and fibronectin treatment leads to generation of dendritic cells with superior endocytic capacity. PLoS One 2010;5(4):e10123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pustylnikov S, Sagar D, Jain P, and Khan Z: Targeting the C-type lectins-mediated host-pathogen interactions with dextran. J Pharm Pharmaceut Sci 2014;17(3):371–392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gopi H, Cocklin S, Pirrone V, et al. : Introducing metallocene into a triazole peptide conjugate reduces its off-rate and enhances its affinity and antiviral potency for HIV-1 gp120. J Mol Recog 2009;22(2):169–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bastian AR, Contarino M, Bailey LD, et al. : Interactions of peptide triazole thiols with Env gp120 induce irreversible breakdown and inactivation of HIV-1 virions. Retrovirology 2013;10(1):153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tabarani G, Thépaut M, Stroebel D, et al. : DC-SIGN neck domain is a pH-sensor controlling oligomerization SAXS and hydrodynamic studies of extracellular domain. J Biol Chem 2009;284(32):21229–21240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Connor RI, Chen BK, Choe S, and Landau NR: Vpr is required for efficient replication of human immunodeficiency virus type-1 in mononuclear phagocytes. Virology 1995;206(2):935–944 [DOI] [PubMed] [Google Scholar]
- 22.Dong C, Janas AM, Wang J-H, et al. : Characterization of human immunodeficiency virus type 1 replication in immature and mature dendritic cells reveals dissociable cis- and trans-infection. J Virol 2007;81(20):11352–11362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Quon CY: Clinical pharmacokinetics and pharmacodynamics of colloidal plasma volume expanders. J Cardiothorac Anesth 1988;2(6):13–23 [Google Scholar]
- 24.Takahara K, Yashima Y, Omatsu Y, et al. : Functional comparison of the mouse DC-SIGN, SIGNR1, SIGNR3 and Langerin, C-type lectins. Int Immunol 2004;16:819–829 [DOI] [PubMed] [Google Scholar]
- 25.Feinberg H, Mitchell DA, Drickamer K, and Weis WI: Structural basis for selective recognition of oligosaccharides by DC-SIGN and DC-SIGNR. Science 2001;294(5549):2163–2166 [DOI] [PubMed] [Google Scholar]
- 26.McFadden K, Fletcher P, Rossi F, et al. : Antiviral breadth and combination potential of peptide triazole HIV-1 entry inhibitors. Antimicrob Agents Chemother 2012;56(2):1073–1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Larsson B, Lalos O, Marsk L, et al. : Effect of intraperitoneal instillation of 32% dextran 70 on postoperative adhesion formation after tubal surgery. Acta Obstet Gynecol Scand 1985;64(5):437–441 [DOI] [PubMed] [Google Scholar]
- 28.Pustylnikov SV: [Research of cytotoxic action of dextrans oxidized with Mn[VII] salts] [Abstract in Russian]. Paper presented at “Fundamental Sciences and Practice,” Tomsk, 2010 [Google Scholar]
- 29.Kaneko T, Kohmoto T, Fukui F, et al. : Acute and chronic toxicity and mutagenicity studies on isomaltooligosaccharides, and the effects on peripheral blood lymphocytes and intestinal microflora. Shokuhin Eiseigaku Zasshi: J Food Hygienic Soc Jpn 1990;31(5):394–403 [Google Scholar]
- 30.Palella FJ, Jr, Baker RK, Moorman AC, et al. : Mortality in the highly active antiretroviral therapy era: Changing causes of death and disease in the HIV outpatient study. JAIDS: J Acquir Immune Defic Syndr 2006;43(1):27–34 [DOI] [PubMed] [Google Scholar]
- 31.Mehvar R. and Shepard TL: Molecular-weight-dependent pharmacokinetics of fluorescein-labeled dextrans in rats. J Pharm Sci 1992;81(9):908–912 [DOI] [PubMed] [Google Scholar]
- 32.Steffansen B, Nielsen CU, Brodin B, et al. : Intestinal solute carriers: An overview of trends and strategies for improving oral drug absorption. Eur J Pharmaceut Sci 2004;21(1):3–16 [DOI] [PubMed] [Google Scholar]
- 33.Ketabi A, Dieleman LA, and Ganzle MG: Influence of isomalto-oligosaccharides on intestinal microbiota in rats. J Appl Microbiol 2011;110(5):1297–1306 [DOI] [PubMed] [Google Scholar]
- 34.Vos AP, M'Rabet L, Stahl B, et al. : Immune-modulatory effects and potential working mechanisms of orally applied nondigestible carbohydrates. Crit Rev Immunol 2007;27(2):97–140 [DOI] [PubMed] [Google Scholar]
- 35.Geijtenbeek TBH, Kwon DS, Torensma R, et al. : DC-SIGN, a dendritic cell-specific HIV-1-binding protein that enhances trans-infection of T cells. Cell 2000;100(5):587–597 [DOI] [PubMed] [Google Scholar]
- 36.Rappocciolo G, Piazza P, Fuller CL, et al. : DC-SIGN on B lymphocytes is required for transmission of HIV-1 to T lymphocytes. PLoS Path 2006;2(7):e70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tran TH, El Baz R, Cuconati A, et al. : A novel high-throughput screening assay to identify inhibitors of HIV-1 gp120 protein interaction with DC-SIGN. J Antivir Antiretrovir 2011;3:49–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pustylnikov S, Sagar D, Jain P, and Khan ZK: Targeting the C-type lectins-mediated host-pathogen interactions with dextran. J Pharm Pharm Sci 2014;17(3):371–392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nguyen DG. and Hildreth JEK: Involvement of macrophage mannose receptor in the binding and transmission of HIV by macrophages. Eur J Immunol 2003;33:483–493 [DOI] [PubMed] [Google Scholar]
- 40.Trujillo JR, Rogers R, Molina RM, et al. : Noninfectious entry of HIV-1 into peripheral and brain macrophages mediated by the mannose receptor. Proc Natl Acad Sci USA 2007;104(12):5097–5102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lopez-Herrera A, Liu Y, Rugeles MT, and He JJ: HIV-1 interaction with human mannose receptor (hMR) induces production of matrix metalloproteinase 2 (MMP-2) through hMR-mediated intracellular signaling in astrocytes. Biochim Biophys Acta 2005;1741(1):55–64 [DOI] [PubMed] [Google Scholar]