Abstract
Previous investigations have demonstrated that activation with the type II interferon, IFN-γ, downregulates alveolar macrophage (AM) phagocytosis of Streptococcus pneumoniae. While these studies have shown clear effects at discrete time points, the kinetics of the macrophage response to IFN-γ over time, with respect to pneumococcal phagocytosis, have not been shown. Here, we describe these kinetics in the murine MH-S AM cell-line, a well-established model useful for investigations of AM phenotype and function. We measure binding and internalizing rates of S. pneumoniae following exposure to increasing durations of physiologic levels of IFN-γ. When MH-S murine alveolar macrophage (mAM) were exposed to IFN-γ for increasing durations of time, from 0 to 6 days before inoculation with the type II S. pneumoniae, D39, exposure for 6 h transiently reduced bacterial binding by 50%, which was temporarily restored at 2 and 3 days of exposure. Bacterial internalization was also reduced shortly following initial exposure, however, internalization continued to fall to less than 5% that of IFN-γ naïve controls after 6 days of exposure. These data may help explain otherwise contradictory reports from the literature regarding timing between infections and reductions in macrophage function.
Introduction
Type I and II IFNs are each recognized as important modulators of the innate immune system, aiding in viral and bacterial and protozoal clearance (Schroder and others 2004). During the early course of an infection, IL-12 and IL-18 promote IFN-γ secretion (Munder and others 2001) from numerous antigen-presenting cells, including monocytes/macrophage, dendritic cells, and natural killer cells, and later in infection from CD4+ T helper and CD8+ cytotoxic lymphocytes (Otani and others 1999; Schroder and others 2004). Once secreted, the type II IFN aids in leukocyte recruitment, upregulation of antigen presentation by both MHC class I and II, differentiation of multiple cell types, and enhances or reduces macrophage function (Schroder and others 2004; Sun and Metzger 2008). While IFN-γ (originally termed “macrophage activating factor”) is widely known to activate macrophage activity, it is also known to reduce alveolar macrophage (AM) detection and phagocytosis of the encapsulated Gram-positive bacterial pathogen Streptococcus pneumonia (the pneumococcus) (Mosser and Handman 1992; Chroneos and Shepherd 1995; Sun and Metzger 2008). In mice, this reduction in phagocytic activity occurs within hours of IFN-γ treatment and is mediated, at least in part, by a reduction in expression of the class A scavenger receptor MARCO (macrophage receptor with collagenous structure) on the surface of the AM (Sun and Metzger 2008), important for pneumococcal surveillance and clearance (Arredouani and others 2004). Reduced phagocytosis may also be mediated by reductions in C-type lectin receptors on the macrophage cell surface, important for detection and killing of S. pneumonia (Chroneos and Shepherd 1995). Here, we sought to phenotypically describe pneumococcal binding and internalization as a function of duration of exposure to IFN-γ in a model system of murine AMs using the well established MH-S murine AM cell-line (Mbawuike and Herscowitz 1989).
Materials and Methods
Cell culture and IFN-γ exposure
The murine alveolar macrophage (mAM) cell line, MH-S (American Type Culture Collection), was used as a model system to investigate the effects of IFN-γ on binding and internalization of Streptococcus pneumoniae. MH-S cells were harvested and quantified using a Countess™ (Invitrogen) automated cell counter with 0.4% trypan blue and plated at 200,000 cells/mL in RPMI 1640 media containing 10% fetal bovine serum and 1% penicillin/streptomycin at 37°C in 5% CO2. For all IFN-γ exposures, plates were seeded with MH-S cells 7 days before phagocytosis assays. Once seeded, cells were allowed to adhere for at least 24 h after which, cells were cultured in media with, or without, 25 ng/mL of recombinant mouse IFN-γ (eBioscience), depending on the designated conditions for the particular wells. For example, cells designated for exposure to IFN-γ for a total of 6 h began receiving media containing IFN-γ at 6.75 days postseeding while cells designated for 6 days of IFN-γ exposure began receiving IFN-γ containing media 24 h postseeding. Because IFN-γ has a relatively short half-life, media were changed (for all wells) daily to ensure consistent levels of IFN-γ during exposure periods. Cells were not passaged during any of the experimental conditions (ie, between initial plating and assays 7 days later). Twenty-four hours before phagocytosis assays, cells were washed 3 times in 1 mL of sterile phosphate-buffered saline (PBS) to remove antibiotics, and antibiotic-free media, with or without IFN-γ treatment, were added.
Bacteria
All phagocytosis assays were performed using the type II encapsulated S. pneumoniae (pneumococcus) strain D39. Bacteria were grown in Todd-Hewitt broth supplemented with 0.5% (w/v) yeast extract (Difco) to an optical density (OD600) of 0.3–0.35, corresponding to mid- to late-log phase growth and an approximate bacterial density of 1×108 colony forming units (CFUs) per mL. Bacteria were then pelleted at 10,000 g and resuspended in 100 μl RPMI for use in phagocytosis assays. For all experiments, the number of bacterial CFUs was verified by serial dilution plating on blood agar plates.
For experiments measuring the phagocytic index (PI), a modified D39 pneumococcal strain was engineered to express the green-fluorescence protein (GFP), referred to hereafter as D39-GFP. Briefly, WT D39 was transformed with the mobilizable plasmid, pMV158, harboring the gene encoding GFP (Nieto and Espinosa 2003). The plasmid confers resistance to tetracycline by constitutive expression of the tetL gene, encoding an energy-dependent tetracycline efflux pump (Hernandez-Arriaga and others 2012) and GFP is under the PM promotor inducible by inoculating the bacterial culture in the presence of 2% maltose. D39-GFP can be quantitated via fluorescent microscopy and measurement of mean fluorescent units.
Phagocytosis assays: multiplicity of infection
Pneumococci were added for phagocytosis at a multiplicity of infection (MOI) of 15 bacteria per MH-S cell. Because IFN-γ reduces replication rates of MH-S cells (see Supplementary Fig. S1A; Supplementary Data are available online at www.liebertonline.com/jir), 2 wells of MH-S cells per experimental condition (where each “condition” is defined as a particular duration of IFN-γ exposure) were plated along with each experiment solely for cell counting before addition of bacteria into the experimental wells to ensure consistent MOIs. Viable cell counts (Supplementary Fig. S1B) were measured in duplicate for each condition using a Countess (Invitrogen) automated cell counter with 0.4% trypan blue, and numbers of bacteria added to experimental wells were adjusted for each condition depending on numbers of live MH-S cells accordingly. Numbers of bacterial CFUs were verified by serial dilution plating on 5% sheep-blood agar plates.
Total phagocytic activity: binding and internalization
Overall phagocytic activity was measured as the ratio of the sum of both bound and internalized bacteria per macrophage using standard phagocytosis assays, as have been previously reported (Zhou and Kobzik 2007). Briefly, IFN-γ exposed or unexposed MH-S cells were cultured with pneumococci at an MOI of 15 for 50 min at 37°C in 5% CO2. Media was then aspirated off and cells washed twice in 1 mL of sterile PBS to remove any unbound bacteria within the supernatant. Cells were gently lysed by incubation with 1% saponin (Sigma) in RPMI 1640 for 15 min at 37°C to release both surface bound and internalized bacteria. Cell lysate was collected and serial dilutions were plated on 5% sheeps-blood agar plates for bacterial CFU enumeration. Three wells per experimental condition were analyzed and all experiments were further performed in triplicate. For statistical analysis, the 9 data points per condition were pooled.
Bacterial internalization
Bacterial internalization was measured using a well established gentamicin protection assay (Tabrizi and Robins-Browne 1993) by first incubating pneumococci with MH-S cells for 50 min and washing with PBS to remove unbound bacteria as described above. After removal of unbound bacteria, cells were incubated for a further 50 min in fresh media containing 120 μg/mL gentamicin sulfate to kill extracellular membrane-bound bacteria that had not yet been internalized. This duration has been shown to be of sufficiently short duration to prevent any significant microbicidal effects of unopsonized encapsulated pneumococcal bacteria that would otherwise confound measurements of internalization (ie, by killing internalized bacteria and thus preventing bacterial enumeration from lysed MH-S cells). Cells were then washed twice in ice-cold PBS before gentle lysis using 1% saponin as described above. Cell lysate was collected and internalized bacteria were enumerated by serial dilution plating on blood agar plates and are reported per MH-S cell. As above, 3 wells per experimental condition were analyzed and experiment was performed in triplicate and the 9 data points were pooled for statistical analyses.
Bacterial binding
Bound bacteria were quantified, indirectly, by subtracting the number of bacterial CFUs measured following gentamicin protection assays (eg, internalized bacteria) from CFUs enumerated in the absence of gentamicin, described above (see “binding and internalization”). Binding activity was calculated as the number of bound bacteria per MH-S cell. Three wells per experimental condition were analyzed and the experiments were performed in triplicate. For statistical analysis, the 9 data points were pooled.
Fluorescent microscopy and the PI
The PI has been described previously (Mancuso and others 1998). Briefly, MH-S cells were seeded as described above, except that wells were lined with 12 mm glass coverslips (No. 1 thickness) before seeding. As above, cells were exposed to varying durations of IFN-γ before inoculation with the GFP-expressing pneumococcus, D39-GFP. Fifty minutes following bacterial inoculation (MOI of 15) and incubation at 37°C in 5% CO2, cells were gently washed thrice with 1 mL ice-cold PBS to remove unbound bacteria immediately before fixing with 4% paraformaldehyde. Coverslips were mounted using ProLong® Gold anti-fade reagent with 4′,6-diamidino-2-phenylindole (DAPI; Invitrogen) and PI was calculated as the fraction of GFP-positive MH-S cells per field (eg, % MH-S cells associated with at least 1 bacterium) multiplied by the mean fluorescence intensity (MFI; representing both bound and internalized bacteria) per cell. The use of GFP-expressing pneumococci allowed MFI quantitation at 100× magnification. Data were analyzed as the mean of at least 5 randomly selected fields for each of 2 replicates per experimental condition, over 3 entirely distinct experiments, resulting in 6 datum per experimental condition. To more closely verify that bacteria were associated with the cells and not simply residual bacteria remaining on the glass coverslip, a subset of samples were stained for actin and high-powered magnification (1,000×) was used to visualize pneumococcal-MH-S association (see Supplementary Fig. S2).
Statistics
All statistical tests were performed within the R statistical computing environment (R version 2.14, R foundation for statistical computing; R Development Core Team). ANOVA plus Dunnett's test were used for hypothesis testing for bacterial CFU results when multiple comparisons were made against the control group. Similarly, single-sample t-tests with Bonferroni correction for multiple comparisons were used for hypothesis testing when ratio of the PI was the outcome. In this case, single sample t-tests were used because standardization of the control to a value of 1, precludes the use of ANOVA and 2-sample t-tests due to zero variance in the comparator (ie, control).
Results
Effects of IFN-γ exposure on pneumococcal binding
We first sought to investigate the dynamics of pneumococcal binding to MH-S murine alveolar macrophage (mAM) given varying durations of exposure to IFN-γ. mAM were cultured in vitro in the presence (treatment) or absence (controls) of increasing durations (6 h–6 days) of exposure to physiologic levels (25 ng/mL) of recombinant IFN-γ. Binding was measured as the difference between total bacteria CFUs remaining following lysis with 1% saponin (bound and internalized bacteria) and CFUs remaining following treatment with gentamicin and lysis with 1% saponin (internalized only; see the Materials and Methods section above). Relative to IFN-γ naïve controls, exposure for only 6 h significantly reduced pneumococcal binding rates of mAM by ∼50% (P<0.05; Fig. 1). Interestingly however, this reduction was transient and pneumococcal adherence following 2 days of exposure were restored to 87% of IFN-γ naïve controls. Following 4 and 6 days of treatment, however, binding rates were ∼60% and of 40% of controls (P<0.05 for each; Fig. 1).
FIG. 1.
Nonlinear effects of IFN-γ exposure on bacterial binding. MH-S cells were seeded and grown in media (changed daily) for 7 days, with varying durations of exposure to IFN-γ (25 ng/mL/day) lasting between 0 and 6 days before inoculation with the type II Streptococcus pneumoniae D39. Bacterial binding was measured as the difference between total bacterial colony forming units (CFUs) internalized and bound (bacteria released from MH-S cells with saponin treatment only) versus bacterial CFUs internalized (gentamicin protection assay to kill extracellular-bound cells followed by saponin treatment to release internalized bacteria). Bacterial CFUs reflect bacteria bound per macrophage (ie, bound CFU/viable MH-S cells). Binding in each condition are then reported as a proportion of the mean CFUs bound to IFN-γ unexposed control cells. Each bar represents pooled results from 3 independent experiments and each experiment examining 3 distinct wells for each condition for a total of 9 data points per condition (depicted as gray filled circles). Boxes represent the interquartile range (IQR) and whiskers extend to 1.5× IQR on either side. Horizontal lines represent the median and the connecting line connects the means. Asterisks represent P<0.05 based on a 2-tailed Dunnett's test, relative to IFN-γ naïve controls.
IFN-γ reduces pneumococcal internalizing capacity
To determine effects of increasing durations of exposure to IFN-γ on pneumococcal internalization, a gentamicin protection assay was performed to kill extracellular bacteria while keeping internalized bacteria alive and viable for bacterial counting after mAM lysis. As opposed to pneumococcal binding, internalization steadily decreased with increasing IFN-γ exposure (Fig. 2; P<0.05 for all time points). As mentioned above (see the Materials and Methods section), this reduction is unlikely a result of enhanced microbicidal activity as the duration of incubation is sufficiently short to prevent any significant micobicidal effects against unopsonized encapsulated pneumococci (Gordon and others 2000). This was also verified by visualizing GFP-expressing bacteria, as described in the Materials and Methods and in the following Results sections. The reduction in internalization over the first 4 days can be accurately described by an exponential decay function, with a decay rate (r) of −0.38 per day of IFN-γ exposure (R2=0.84).
FIG. 2.
Bacterial internalization as a function of exposure to IFN-γ. MH-S cells were seeded and grown in media (changed daily) for 7 days, with varying durations of exposure to IFN-γ (25 ng/mL/day) lasting between 0 and 6 days before inoculation with the type II Streptococcus pneumoniae D39. Bacterial internalization was measured as the number of internal bacterial CFUs released from MH-S cells after treatment with gentamicin and saponin. Bacterial internalization was quantified as bacteria internalized per macrophage and are reported here as proportions of the mean CFUs internalized in IFN-γ unexposed cells. Figure layout is as described in Figure 1 except that asterisks represent P values <0.01.
IFN-γ diminishes phagocytosis: evaluated with the PI
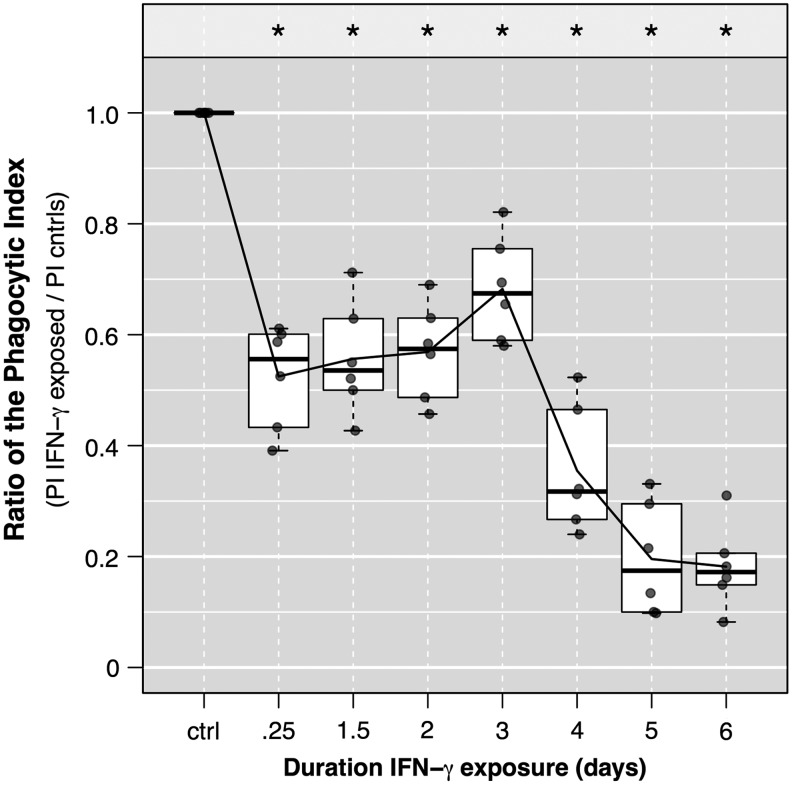
To confirm our findings above, which utilized mAM lysis and serial dilution plating of pneumococci, we performed a series of similar experiments, measuring results using fluorescent microscopy and calculations of the PI (see the Materials and Methods section above). GFP-expressing pneumococci were added to MH-S cells and subsequently fixed and stained for relative quantitation of pneumococci via MFI (see Supplementary Fig. S3, eg, images). Importantly, the PI here is a measure of AM capacity to associate with bacteria (including binding and internalization). We report our findings as a ratio of the phagocytic index (rPI) of IFN-γ exposed versus IFN-γ unexposed (control) MH-S cells (Fig. 3). After 6 h of exposure to IFN-γ, the rPI fell from 1 (standardized control) to 0.52 (P<0.01). Similar to the binding results reported above, the PI of the exposed mAM recovered to nearly 70% that of controls and dropped to ∼35% and ∼20% cells exposed for 4 and 6 days, respectively (P<0.001 for each). That the phagocytic capacity, determined by the rPI here, resemble effects of IFN-γ on pneumococcal binding (rather than internalization) is as expected because AM cells are generally more efficient at binding than internalizing encapsulated pneumococci, and thus the PI is driven by bound bacteria.
FIG. 3.
Changes in the phagocytic index (PI) with extended IFN-γ exposure. MH-S cells were seeded and grown in media (changed daily) for 7 days, with varying durations of exposure to IFN-γ (25 ng/mL/day) lasting between 0 and 6 days before inoculation with a green-fluorescence protein (GFP) expressing D39 pneumococcus [multiplicity of infection (MOI) of 15]. Fifty minutes following bacterial inoculation, cells were gently washed thrice with 1 mL ice-cold phosphate-buffered saline (PBS) to remove unbound bacteria immediately before fixing with 4% paraformaldehyde. The PI was calculated as the fraction of GFP-positive MH-S cells per field (eg, % MH-S cells associated with at least 1 bacterium) multiplied by the mean fluorescence intensity (MFI; representing both bound and internalized bacteria) per cell. Data are representative of 2 independent experiments, and each experimental result calculated by enumerating at least 5 randomly selected fields from each of 3 replicate wells. Data were normalized for each experiment to the PI of the control cells and plotted (filled gray dots). Boxes represent the IQR and whiskers extend to 1.5× IQR on either side. Horizontal lines represent the medians and the connecting line connects the means. Asterisks represent P<0.00; two-tailed single sample t-test with Bonferroni correction for multiple comparisons.
Discussion
Multiple reports have described a role for IFN-γ in the downregulation of important AM cell surface receptors required for proper detection and phagocytosis of Streptococcus pneumonia and other bacterial pathogens. For example, Sun and Metzger demonstrated that even short durations of IFN-γ exposure can significantly reduce expression of the class A scavenger receptor, MARCO, one of numerous factors important for proper pneumococcal clearance (Kraal and others 2000; Sun and Metzger 2008). Similarly, IFN-γ has been implicated in the reduction of the C-type lectin receptors (eg, the mannose receptor), also important for pneumococcal phagocytosis (Chroneos and Shepherd 1995) and Mosser and Handman (1992) too have shown decreased phagocytosis of leishmania promastigotes in response to IFN-γ. While these findings seem in contrast to the better-known role of IFN-γ in the activation of resting macrophage (Mosser and Edwards 2008), Mosser demonstrates that activated macrophage, while more apt to spread out and have higher pinocytic rates, are not more phagocytic than resident unactivated macrophage due to downregulation of important surface receptors required for phagocytosis (Mosser 2003). Further, once internalized, Trost and others (2009) and Yates and others (2007) discovered that IFN-γ exposure delays lysosomal fusion and reductions of hydrolytic and proteolytic activities within the phagosome. An interesting interpretation, Trost hypothesized that such a delay in lysosomal fusion, while seemingly at odds with activation of macrophage functions, could ultimately enhance antigen presentation (Trost and others 2009).
The present work adds to these studies by providing insight into the kinetics of the response to extended IFN-γ over time, rather than at a single timepoint. Although this work is limited by its focus on phenotype rather than mechanism, it is our intent that such an understanding of the kinetics of bacterial binding and internalization will aid future studies to better understand and elucidate underlying mechanisms of various time-dependent disease processes, particularly with regard to co-infections that are known to result in excess pneumococcal bacterial infections following virus-mediated secretion of IFN-γ (McCullers 2006; Sun and Metzger 2008).
In agreement with the reports mentioned above, we found that IFN-γ reduces pneumococcal phagocytosis by the MH-S murine AM cell-line. Intriguingly, we found that effects on binding, versus internalization, are distinct and nonlinear. Exposure to IFN-γ resulted in steady decay in the rate of pneumococcal internalization, while binding recovered to near control levels after 2 and 3 days of exposure following an initial decrease shortly following initial exposure. As mentioned, we cannot conclude from this study the mechanisms underlying the transient recovery in binding capacity. However, this finding agrees with previous investigations by Metzger and Sun who found, in vivo, that while internalization is significantly reduced within 24 h of IFN-γ exposure, excess susceptibility to bacterial disease is often detected only 4–6 days following initial increases in IFN-γ. In this case, one could imagine that although internalization may be reduced, near normal binding rates may be sufficient to temporarily stave off severe excess bacterial disease until after both internalization and binding have fallen to below protective levels (Chow and others 2010). Such a finding would be further supported if macrophage, like neutrophils demonstrated a strong ability to kill bound pathogens in an extracellular fashion, without the need for ingestion.
Recently, Chow and others (2010) discovered macrophage extracellular trap (MET) formation following treatment with statins in the RAW 264.7 murine macrophage cell line. They report that METs led to normal bacterial clearance following statin use that was coincident with reductions in bacterial internalization and suggest that MET formation induced by statins provides a mechanism whereby normal bacterial binding and clearance may persist in the absence of internalization. It is possible that excessive activation following extended exposure to IFN-γ may induce development of MET-like phenotypes in AMs that could allow binding and bacterial killing in the absence of internalization. Indeed, this is a phenotype (see Supplementary Fig. S4) that we have detected in our investigations (data not shown) but requires verification.
Our findings are in agreement with, and support the numerous previous reports that have demonstrated that IFN-γ exposure reduces pneumococcal clearance both in vivo and in vitro. However, this work must be limited here to the MH-S AM cell line. Although this cell line is well described, with a rich history of use for in vitro investigations that have agreed with in vivo findings, our results should be confirmed within in vivo or ex vivo systems.
Supplementary Material
Acknowledgments
We would like to thank Frank L. Harris (Department of Pediatrics, Emory University School of Medicine) for his assistance with laboratory work. Financial Support for this work came from a Burroughs Wellcome Fund “Molecules to Mankind” grant to M.J.M.
Author Disclosure Statement
All authors declare that no competing financial interests exist.
References
- Arredouani M, Yang Z, Ning Y, Qin G, Soininen R, Tryggvason K, Kobzik L. 2004. The scavenger receptor MARCO is required for lung defense against pneumococcal pneumonia and inhaled particles. J Exp Med 200(2):267–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow OA, von Kockritz-Blickwede M, Bright AT, Hensler ME, Zinkernagel AS, Cogen AL, Gallo RL, Monestier M, Wang Y, Glass CK, Nizet V. 2010. Statins enhance formation of phagocyte extracellular traps. Cell Host Microbe 8(5):445–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chroneos Z, Shepherd VL. 1995. Differential regulation of the mannose and SP-A receptors on macrophages. Am J Physiol 269(6 Pt 1):L721–L726 [DOI] [PubMed] [Google Scholar]
- Gordon SB, Irving GR, Lawson RA, Lee ME, Read RC. 2000. Intracellular trafficking and killing of Streptococcus pneumoniae by human alveolar macrophages are influenced by opsonins. Infect Immun 68(4):2286–2293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Arriaga AM, Espinosa M, del Solar G. 2012. Fitness of the pMV158 replicon in Streptococcus pneumoniae. Plasmid 67(2):162–166 [DOI] [PubMed] [Google Scholar]
- Kraal G, van der Laan LJ, Elomaa O, Tryggvason K. 2000. The macrophage receptor MARCO. Microbes Infect 2(3):313–316 [DOI] [PubMed] [Google Scholar]
- Mancuso P, Standiford TJ, Marshall T, Peters-Golden M. 1998. 5-Lipoxygenase reaction products modulate alveolar macrophage phagocytosis of Klebsiella pneumoniae. Infect Immun 66(11):5140–5146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mbawuike IN, Herscowitz HB. 1989. MH-S, a murine alveolar macrophage cell line: morphological, cytochemical, and functional characteristics. J Leukoc Biol 46(2):119–127 [DOI] [PubMed] [Google Scholar]
- McCullers JA. 2006. Insights into the interaction between influenza virus and pneumococcus. Clin Microbiol Rev 19(3):571–582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosser DM. 2003. The many faces of macrophage activation. J Leukoc Biol 73(2):209–212 [DOI] [PubMed] [Google Scholar]
- Mosser DM, Edwards JP. 2008. Exploring the full spectrum of macrophage activation. Nat Rev Immunol 8(12):958–969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosser DM, Handman E. 1992. Treatment of murine macrophages with interferon-gamma inhibits their ability to bind leishmania promastigotes. J Leukoc Biol 52(4):369–376 [DOI] [PubMed] [Google Scholar]
- Munder M, Mallo M, Eichmann K, Modolell M. 2001. Direct stimulation of macrophages by IL-12 and IL-18—a bridge built on solid ground. Immunol Lett 75(2):159–160 [DOI] [PubMed] [Google Scholar]
- Nieto C, Espinosa M. 2003. Construction of the mobilizable plasmid pMV158GFP, a derivative of pMV158 that carries the gene encoding the green fluorescent protein. Plasmid 49(3):281–285 [DOI] [PubMed] [Google Scholar]
- Otani T, Nakamura S, Toki M, Motoda R, Kurimoto M, Orita K. 1999. Identification of IFN-gamma-producing cells in IL-12/IL-18-treated mice. Cell Immunol 198(2):111–119 [DOI] [PubMed] [Google Scholar]
- Schroder K, Hertzog PJ, Ravasi T, Hume DA. 2004. Interferon-gamma: an overview of signals, mechanisms and functions. J Leukoc Biol 75(2):163–189 [DOI] [PubMed] [Google Scholar]
- Sun K, Metzger DW. 2008. Inhibition of pulmonary antibacterial defense by interferon-gamma during recovery from influenza infection. Nat Med 14(5):558–564 [DOI] [PubMed] [Google Scholar]
- Tabrizi SN, Robins-Browne RM. 1993. Elimination of extracellular bacteria by antibiotics in quantitative assays of bacterial ingestion and killing by phagocytes. J Immunol Methods 158(2):201–206 [DOI] [PubMed] [Google Scholar]
- Trost M, English L, Lemieux S, Courcelles M, Desjardins M, Thibault P. 2009. The phagosomal proteome in interferon-gamma-activated macrophages. Immunity 30(1):143–154 [DOI] [PubMed] [Google Scholar]
- Yates RM, Hermetter A, Taylor GA, Russell DG. 2007. Macrophage activation downregulates the degradative capacity of the phagosome. Traffic 8(3):241–250 [DOI] [PubMed] [Google Scholar]
- Zhou H, Kobzik L. 2007. Effect of concentrated ambient particles on macrophage phagocytosis and killing of Streptococcus pneumoniae. Am J Respir Cell Mol Biol 36(4):460–465 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.