Abstract
Deep brain stimulation (DBS) is a well-established treatment modality for movement disorders. As more behavioral disorders are becoming understood as specific disruptions in neural circuitry, the therapeutic realm of DBS is broadening to encompass a wider range of domains, including disorders of compulsion, affect, and memory, but current understanding of the cellular mechanisms of DBS remains limited. We review progress made during the last decade focusing in particular on how recent methods for targeted circuit manipulations, imaging and reconstruction are fostering preclinical and translational advances that improve our neurobiological understanding of DBS’s action in psychiatric disorders.
INTRODUCTION
There is increasing awareness that ‘circuitopathies’, dysfunctions in brain circuits characterized by abnormal patterns of electrical activity and oscillations, are responsible for the signs and symptoms of neurological and psychiatric disorders. This has coincided with a rapid shift in the conceptualization of novel treatment strategies, away from brain-wide interventions based on pharmacology, and towards an upcoming generation of pathway-focused and device-based therapeutics or ‘electroceuticals’ [1]. These approaches aim to reprogram faulty circuits by capitalizing on our greater understanding of the brain’s cellular architecture and the mechanisms of activity-dependent neuroplasticity. Deep Brain Stimulation (DBS) has been the prototype and is currently the most clinically-advanced of such approaches. This technique, which emerged in the 1980’s, has arguably served as one of the triggers for the aforementioned shift. DBS refers to the process of delivering an electrical current to a precise location in the brain using surgically implanted chronic electrodes [2,3].
The use of DBS in Parkinson’s Disease (PD) and other neurological disorders has thus far been the main application of this technology. Chronic high-frequency DBS for treatment of movement disorders was pioneered in the early 1990s [2,4], and stimulation of the subthalamic nucleus (STN), global pallidus (GPi), and ventral intermediate nucleus (VIM) are now common procedures for treatment-resistant PD and essential tremor [3,5]. Nearly 100,000 patients have been implanted with DBS devices in the US [3] and this number is growing at a rate of 8,000–10,000 patients per year [6].
In the early 2000’s, the success of DBS for movement disorders coupled with an increasing understanding of the circuitry underlying mental disorders spurred initial investigations into the efficacy of DBS in psychiatry. This review will provide an overview of the principles of DBS action in this context, summarize the progress made during the last decade in this area and discuss the emerging understanding of the circuit, cellular and molecular mechanisms underlying its therapeutic activity.
GENERAL PRINCIPLES OF DBS ACTION: STILL MANY OPEN QUESTIONS
A/Stimulatory versus inhibitory effects on cell firing at the site of stimulation
DBS stimulates a spherical volume of tissue around the electrode [7], and the effects of this stimulation can vary regionally depending on the molecular characteristics of local neurons or glial cells, which determine their passive membrane properties and compositions of voltage-sensitive ion channels [2]. Accordingly, the response of individual cell bodies in the stimulated region is typically phase-locked to stimulation but varies with regard to the proportion of cells increasing and decreasing their firing rate [2,3,8]. Potential mechanisms for DBS-induced inhibition of cell bodies include depolarization block, inactivation of Na+ channels, presynaptic depression or depletion of excitatory afferents, and stimulation of inhibitory afferents [3].
B/Modulation of cell bodies and dendrites versus axons
Because the chronaxie of a myelinated axon is typically orders of magnitude lower than for cell bodies or dendrites (making the former more excitable), DBS may exert its effects predominantly by modulating axons that are afferent to, efferent from, or passing through the site of stimulation [2,9]. Accordingly, preclinical studies using optogenetics to dissect the action of DBS have shown that direct optical stimulation or inhibition of neuronal cell bodies at the site of electrode may not reproduce therapeutic effect of DBS, while direct optical stimulation of afferent axons to this region does so [10]. This axonal mode of action explains the paradoxical finding that cell bodies in a stimulated nucleus can be inhibited by DBS, while output from this nucleus increases in projection areas [7]. Accordingly, DBS still maintains its therapeutic activity in certain preclinical models in the presence of lesions that ablate all cell bodies at the site of stimulation, but spare fibers of passage [11].
C/Local versus distal effects
DBS-induced changes outside the area of stimulation are relatively less well-studied. Electrophysiological and imaging studies have revealed that DBS simultaneously modulates blood flow and electrical oscillations across many brain regions distal to the site of stimulation, through both orthodromic and antidromic transmission [3]. For example, in PD, STN stimulation can reverse pathological low-frequency (~9 Hz) single-unit oscillations in the global pallidus externa (GPe) and substantia nigra reticulata (SNr) by entraining neurons in the circuit to the stimulation frequency [12], and can modify the firing probability of cortical neurons through antidromic frequency jamming, reducing pathological cortical beta rhythms [13].
The normalization of aberrant patterns of electrical and metabolic activity in connected regions by DBS may reflect at least in part an effect on neurotransmitter release. For example, NAc DBS has been shown to drive striatal dopamine release in patients and animals [14], and D2 receptor antagonism abolished the effect of NAc DBS on compulsive feeding behaviors in obese mice [15]. Similarly, in depression models, preclinical studies have shown that vmPFC DBS drives hippocampal serotonin (5-HT) release [11], and that serotonin depletion abrogates the antidepressant-like effect of DBS [11,16].
D/Neurons versus glia
In addition to neurons, glia may play an important role in the response to DBS. Two main types of glial cells have been implicated: astrocytes and microglia. Astrocytes are prime candidates as they propagate calcium waves and form a tripartite synapse together with neuronal synapses. Gliotransmitters and growth factors released from astrocytes are thus likely to mediate, at least in part, the activity of DBS in psychiatric illness. Astrocyte-derived adenosine released during DBS and acting at A1 receptors on neurons was found necessary and sufficient for the effect of thalamic DBS on essential tremor [17] and preliminary evidence suggests a similar mode of action in preclinical models of depression [18]. Not only can astrocytes respond to the electrical changes induced by DBS, but the microlesions resulting from the implantation of the stimulating electrode also produce inflammation and reactive gliosis [19], a known source of induced multipotent stem cells in the injured brain. The presence of these cells may contribute to neurotrophic responses and circuit reorganization [20]. Furthermore, cytokines produced by microglia at the site of implantation also appear to participate in the therapeutic action of DBS through effects on endothelial cells. Interestingly, postmortem tissue from PD patients treated with STN DBS showed lower densities of activated microglia and increased microvasculature in the STN compared to control PD patients [21].
F/Acute versus chronic effects
Most mechanistic studies to date have focused on the effects of acute DBS (Figure 1A). Although this approach has validity in the context of PD, where effects on motor deficits are observed immediately, DBS is always applied chronically in clinical settings, and long-term effects on connected networks are beginning to be uncovered in most clinical applications (Figure 1B). For example, in PD, a longitudinal case study comparing brain structural and functional connectivity after 5 months of DBS showed localized structural changes in sensory-motor, prefrontal/limbic, and olfactory brain regions and an increased nodal efficiency [22].
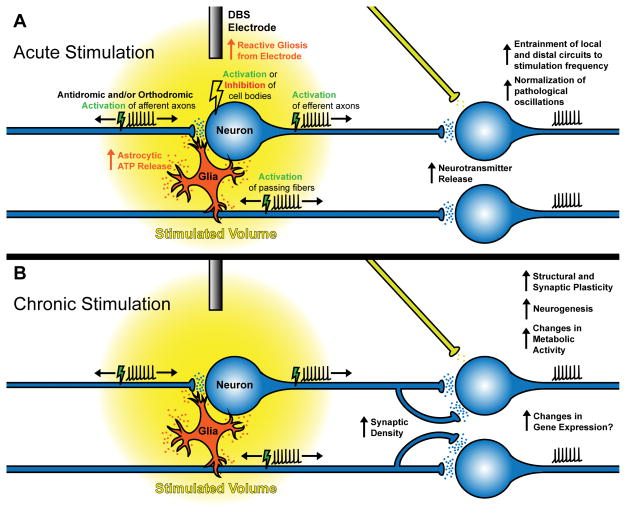
Figure 1. General cellular mechanisms of DBS.
(A) Acute stimulation results in complex effects in local, upstream, and downstream circuits. Insertion of the electrode itself promotes an inflammatory response resulting in reactive gliosis over the course of days. Upon stimulation, DBS stimulates neural cell bodies, axons, and glia, entraining neural activity to the stimulation frequency. Effects on cell bodies are variable and both activation and inhibition of firing rates have been reported. DBS preferentially modulates myelinated axons rather than cell bodies, resulting in antidromic and/or orthodromic stimulation of afferent axons, efferent axons, and passing fibers. How and when antidromic vs. orthodromic activation is elicited is unknown. Modulation of upstream and downstream projections can normalize pathological oscillations in distal regions by entraining activity to the stimulation frequency (frequency jamming), and can result in enhanced neurotransmitter release. (B) The effects of chronic stimulation are less well-understood but neuroplastic mechanisms are evident. Stable metabolic changes and synaptic plasticity occur in the stimulated area and distally modulated regions, as well as neurogenesis and progenitor proliferation. Structural plasticity in the form of changes in intrinsic excitability, synaptic density, and synaptic reorganization can occur as well, possibly driven by activity-induced changes in gene expression. These adaptations may reinforce the effects of acute stimulation.
DISEASE-SPECIFIC MECHANISMS OF DBS IN PSYCHIATRIC DISORDERS
A/Obsessive Compulsive disorders (OCD)
OCD was the first non-motor disorder treated with DBS. It is a serious neuropsychiatric disorder characterized by persistent intrusive anxious thoughts (obsessions) and unwanted repetitive ritualistic behaviors (compulsions) that often have devastating effects on a patient’s life. A quarter of all patients remain resistant to first line treatments (cognitive-behavioral therapy and pharmacotherapy with serotonin reuptake inhibitors). The application of DBS in OCD was pioneered by Bart Nuttin and colleagues in 1999 as a reversible alternative to capsulotomy, a last-resort surgical intervention which permanently disconnects the cingulate cortex by severing the fiber tracts running from the thalamus to the frontal lobe [23].
The current ‘habit hypothesis’ of OCD places emphasis on the pathophysiological role of cortico-striato-thalamo-cortical (CSTC) loops implicated in the acquisition of automatic behaviors [24,25]. To date, most clinical trials of DBS for OCD have focused on stimulation of the anterior limb of the internal capsule in the ventral striatum (VC/VS) [26], the adjacent Nucleus Accumbens (NAc) [27], and the STN [28], which are three nodes in the CSTC “habit” circuit [29]. Although DBS at these sites clearly improves anxiety and compulsions, it does so without affecting threat response of patients in experimental paradigms such as conditioned startle. This confirms the dissociation between substrates of OCD and circuits classically involved in the expression of fear/anxiety [30]. Nevertheless, several animal and human studies have showed consistent effects of ventral striatal DBS on prefrontal cortical circuits implicated in the extinction of fear and addictive behaviors [31].
Single-unit recording studies in OCD patients have revealed increased frequency and variability in the rates of firing of medium spiny neurons (MSNs) in the ventral striatum suggestive of reduced interneuron function and cortico-striatal hyperconnectivity [25]. Important advances in understanding the cellular bases of this dysconnectivity have derived from the recent studies using targeted circuit photomanipulation with optogenetics and genetically engineered mouse models of OCD. Repeated, but not single sessions of optogenetic low-frequency stimulation of corticostriatal pathway produced aberrant repetitive grooming behaviors in naïve mice that persisted for 2 weeks post-stimulation [32], providing a proof of concept that activity-induced maladaptive plasticity in certain corticostriatal networks is sufficient to trigger OCD-like symptoms in normal mice. Similarly, low-frequency optical stimulation of orbitofrontal cortex neurons projecting to the VS normalized compulsive grooming behaviors in the Sapap3 knockout mouse model of OCD, and normalized VS medium spiny neuron hyperactivity by restoring defective intrastriatal feed-forward inhibition by fast-spiking interneurons [33]. In line with this mechanism of action, human DBS attenuated frontal low-frequency oscillations pathologically occurring in response to symptom-inducing stimuli and decreased PFC-NAc connectivity in a manner that strongly correlated with symptom reduction [34]. Unlike the rapid beneficial effects of optogenetic manipulation in Sapap3 knockout mice, OCD symptoms in patients take weeks to months to respond to DBS [35], indicating a possible requirement for neuroplasticity and/or neurogenesis in its therapeutic mechanism. Preclinical studies have shown that high-frequency stimulation of the dorsal portion of the NAc with DBS enhances extinction memory in rats, while upregulating plasticity markers such as pERK [31] and BDNF [36] in the medial prefrontal cortex, and that NAc DBS can increase dentate gyrus neurogenesis [37]. Interestingly, as with compulsive behavior in OCD, NAc DBS can also facilitate the extinction of compulsive drug seeking [38] and compulsive eating, as recently reported in a model of diet-induced binge eating in obese mice [15]. D2, but not D1 dopamine receptor antagonism abolished this effect, suggesting a role for dopamine in the mechanism of DBS in this model.
In sum, these studies indicate that NAc and VC/VS DBS promote complex neuroplastic alterations that de-potentiate overactive corticostriatal circuits in part through the strengthening of feed-forward inhibition in the striatum (Figure 2A).
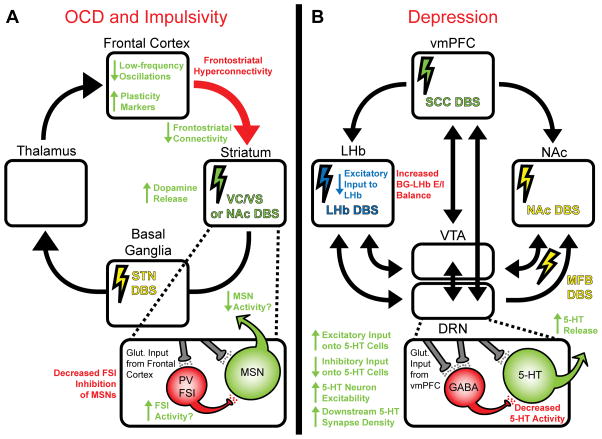
Figure 2. Disease-specific cellular mechanisms of DBS for OCD and depression.
Disease-related deficits are indicated in red text, DBS targets are indicated by colored lightning bolts, and potential compensatory therapeutic effects of specific DBS targets are indicated by color-matched text and arrows. (A) Cortico-striato-thalamo-cortical (CSTC) loop implicated in OCD, which is characterized by pathological frontostriatal hyperconnectivity secondary to a decreased activity of parvalbumin (PV) positive fast-spiking interneurons (FSIs) which monosynaptically inhibit striatal medium spiny neurons (MSNs). Striatal (VC/VS or NAc) DBS has been shown to reduce frontostriatal hyperconnectivity, reduce pathological low-frequency oscillations, release striatal dopamine, and increase plasticity markers such as BDNF in the cortex. It is possible that striatal DBS may also compensate for the cellular deficits in FSIs. STN DBS is also utilized for OCD but its cellular mechanisms are less well-studied. (B) Circuit implicated in depression. In the CSDS model of depression, we have reported several alterations of dorsal raphe nucleus (DRN) microcircuitry that lead to decreased 5-HT output, namely, an intrinsic hypoexcitability of 5-HT neurons and a sensitized feedforward inhibition of serotonin (5-HT) neurons driven by the vmPFC and relayed by hyperexcitable DRN GABAergic interneurons which monosynaptically inhibit 5-HT neurons. vmPFC DBS in rodents, presumably through desensitization of the vmPFC-DRN disynaptic inhibitory circuit, restores 5-HT output and increases 5-HT synapse density in projections regions innervated by the DRN. In MDD patients and in the learned helplessness rodent model of depression, the LHb, a nucleus that signals aversion, is overactive and represses the activity of the VTA and DRN. By depleting afferents from the basal ganglia (BG) which drive LHb hyperactivity, LHb DBS dishinibits the VTA and DRN. MFB DBS directly stimulates the output from the VTA and DRN in projection areas by stimulating axons fibers transiting towards the forebrain via this bundle. DBS of the NAc and VC/VS also reverses depression symptoms in humans and animals, possibly by stimulating afferents from the VTA and DRN, although the cellular mechanisms have not been as extensively studied.
B/Memory
DBS for memory enhancement is in very early stages; the only studies conducted so far have involved case reports or very small sample sizes. Serendipitous observation of promnesic effects upon application of DBS to the hypothalamus/fornix of a patient treated for obesity [39], has prompted more systematic investigations of this target for the treatment of memory disorders. Results of a Phase I trial of hypothalamus/fornix DBS for Alzheimer’s were inconclusive regarding efficacy [40]. High frequency stimulation of the entorhinal cortex (EC), the source of a major excitatory input to the dentate gyrus, was shown to improve spatial memory in epilepsy patients [41] and in rodents [42]. Mechanisms for these memory improvements have not been studied extensively, but EC DBS may mediate its effects partly by stimulating the proliferation of Type 2 progenitors and affecting the integration of newborn neurons into functional circuits. Supporting a causal role, enhancement of dentate gyrus neurogenesis followed the time-course of memory improvement, and administration of a DNA alkylating agent (TMZ) prevented both DBS-induced neurogenesis and memory improvements [42].
C/Depression
Major Depression (MDD) is a heterogeneous multidimensional syndrome characterized by cognitive, affective and neurovegetative deficits. Three well-characterized core cognitive dysfunctions in MDD are 1/the biased processing of negatively valenced emotional information, 2/an inability to control mood fluctuations and 3/anhedonia (a reduced ability to experience pleasure or interest). As in OCD, these dimensions have been linked to dysconnectivity within the distributed CSTC network. One node in this circuit that reliably maps with depressed mood and responses to antidepressants is the subcallosal cortex (SCC) that comprises the subcallosal cingulate gyrus and adjacent ventromedial prefrontal cortex. After extensively characterizing the dysconnectivity of the SCC and CSTC circuit in depressed patients [43] and their engagement during sadness in normal subjects, Mayberg and colleagues provided the initial proof of concept for the efficacy of SCC DBS in treatment-resistant depressive patients [44]. Although these first clinical studies led to promising results, a more recent, larger controlled multicenter trial was recently discontinued after futility analyses revealed inefficacy [45]. Failure is likely attributable to variability of implantation sites since the trial did not use state of the art fMRI mapping prior to implanting the electrodes. Recently, probabilistic tractographic analyses have identified three key myelinated fiber bundles whose contact with the electrode is required to obtain positive response to SCC DBS and which point to selected downstream cortical and subcortical targets [46].
Several other stimulation sites throughout the brain have been tested clinically and were also found to produce mood improvement in refractory MDD patients; there are now almost as many validated DBS targets as there have been clinical studies in MDD [47]. Overall, the published response rate to DBS therapy is 40–70%. Three of the most consistent targets include the NAc/VC/VS, medial forebrain bundle (MFB), and lateral habenula (LHb), which are all coupled (positively or negatively) to the reward circuitry [48]. Cellular mechanisms of DBS for depression at these targets and the SCC are summarized in Figure 2B.
To more precisely investigate mechanisms of SCC DBS, a growing number of preclinical studies in rodents have explored the neurobiological mechanisms underlying the behavioral activity of DBS of the ventromedial prefrontal cortex (vmPFC), which is considered the rodent analog of the human SCC. vmPFC DBS results in antidepressant-like effects in several rodent models sensitive to chemical antidepressants [11,16,49].
Mapping of immediate early genes reveals that vmPFC DBS engages neurons in multiple regions distal to the site of stimulation, including the amygdala, the piriform/insular cortices and monoaminergic nuclei [49,50]. One vmPFC downstream target that is activated by DBS and has been consistently implicated in mediating DBS effects is the serotonergic dorsal raphe nucleus (DRN) in the brainstem. Viral tracing and optogenetic studies from our lab and others have demonstrated the existence of a phylogenetically-conserved monosynaptic vmPFC-DRN pathway, which is likely modulated by DBS. vmPFC DBS induces 5-HT release in projection areas [11], and intact raphe serotonergic function is necessary for the antidepressant-like and anti-anhedonic effects of vmPFC DBS [11,16]. These results suggest that cortical DBS recruits similar neurotransmitter systems to pharmacological antidepressants, but does so in a more efficient and focalized manner, by engaging only selected subsets of projections within the 5-HT system.
The other clinically-effective DBS targets in the reward system (NAc, MFB and LHb) may modulate depression symptoms through a partly overlapping network. Indeed, the NAc receives strong serotonergic and dopaminergic inputs from the DRN and VTA respectively, whose axons all travel via the MFB [51]. The DRN is the strongest source of (serotonergic and glutamatergic) synaptic inputs to the VTA [52]. The LHb, which is hyperactive in MDD, acts as a ‘brake’ on the activity of both VTA and DRN neurons. LHb DBS disinhibits these regions by depleting excitatory projections from the basal ganglia that drive LHb hyperactivity [53–55].
The therapeutic latency of SCC DBS for MDD is typically on the order of weeks to months [56], with the number of responders increasing progressively with the duration of treatment, while discontinuation typically results in the return of symptoms over one to six weeks [48], suggesting the importance of activity-dependent plasticity in the mechanism of DBS. Several cellular-level and network-level plasticity signatures have been shown to be associated with this therapeutic effect. Increased paired-pulse depression, an electrophysiological index of presynaptic plasticity, was reported in patients after application of high-frequency stimulation in the SCC, which predicted treatment response at 6 months [57]. Similarly, some EEG signatures of network connectivity were sustained after turning DBS off and predicted response to DBS [58]. Collectively these findings suggest that long term DBS induces structural and functional neuroplastic changes that normalize pathological activity in distributed networks.
Top-down disinhibition of DRN 5-HT neurons as a mechanism of chronic DBS in MDD?
To more precisely identify plasticity mechanisms implicated in the activity of repeated vmPFC DBS, we recently used transgenic mice to conduct cell type-specific investigations in the chronic social defeat stress (CSDS) model of depression. We found that 1 week of DBS was sufficient to fully restore social approach behaviors in vulnerable mice and normalize CSDS-induced structural and physiological maladaptive plasticity in the DRN. Specifically, vmPFC DBS enhanced the excitatory drive of 5-HT neurons, reduced inhibitory input, altered dendritic arborization, and restored the density of downstream serotonergic synapses which were reduced by CSDS [49]. DBS thus restores 5-HT output and reinforces acute DBS-induced brain-wide 5-HT release. Further investigations will be necessary to determine whether such neuroplastic adaptations are necessary or sufficient for the behavioral effect of DBS.
CONCLUSION
While DBS has demonstrated efficacy across many disorders, the rationale for selecting targets in each of these therapeutic areas remains based on minimal (or no) prior mechanistic studies [2,3]. This is particularly true for the newest applications of DBS such as memory enhancement. Going forward, preclinical studies will continue to be a powerful tool for answering mechanistic and dosimetry questions, although limitations of non-human disease models and cross-species neurocircuitry differences must be taken into account. Critical advances are expected in the next few years with the increasing availability of closed-loop interfaces combining stimulation/recording/electrochemical sensing, MRI compatible systems that may allow for the understanding of the effects of DBS in real time, and noninvasive methodologies such as transcranial magnetic stimulation and transcranial direct current stimulation. Finally, the emergence of novel optogenetic tools to drive circuits with photostimulation at the high frequencies used in DBS promises to offer opportunities to better dissect the role of activity-dependent neurophysiological adaptations in the actions of DBS. Developing an understanding of circuit and cellular level mechanisms of DBS will be essential to inform patient selection, improve efficacy, and potentially allow for other, less-invasive therapies to replace the function of DBS.
Highlights.
Deep Brain Stimulation (DBS) is an invasive form of neuromodulation well-established for the treatment of movement disorders.
Growing investigational uses of DBS encompass disorders of mood, compulsion and cognition.
DBS has neurophysiological effects both at the site of stimulation and in distal distributed networks, and promotes neuroplastic circuit reorganization.
DBS potentially treats compulsions by reversing hyperconnectivity in the cortico-striatal “habit” network.
DBS potentially restores mood by disinhibiting monoaminergic systems.
Acknowledgments
This work was supported by a grant from the National Institute of Mental Health (MH087581) to O.B.
References
- 1.Famm K, Litt B, Tracey KJ, Boyden ES, Slaoui M. Drug discovery: a jump-start for electroceuticals. Nature. 2013;496:159–161. doi: 10.1038/496159a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Perlmutter JS, Mink JW. Deep brain stimulation. Annu Rev Neurosci. 2006;29:229–257. doi: 10.1146/annurev.neuro.29.051605.112824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Agnesi F, Johnson MD, Vitek JL. Deep brain stimulation: how does it work? Handb Clin Neurol. 2013;116:39–54. doi: 10.1016/B978-0-444-53497-2.00004-8. [DOI] [PubMed] [Google Scholar]
- 4.Benabid AL, Pollak P, Gervason C, Hoffmann D, Gao DM, Hommel M, Perret JE, de Rougemont J. Long-term suppression of tremor by chronic stimulation of the ventral intermediate thalamic nucleus. Lancet. 1991;337:403–406. doi: 10.1016/0140-6736(91)91175-t. [DOI] [PubMed] [Google Scholar]
- 5.Miocinovic S, Somayajula S, Chitnis S, Vitek JL. History, applications, and mechanisms of deep brain stimulation. JAMA Neurol. 2013;70:163–171. doi: 10.1001/2013.jamaneurol.45. [DOI] [PubMed] [Google Scholar]
- 6.Ponce FA, Lozano AM. Deep brain stimulation state of the art and novel stimulation targets. Prog Brain Res. 2010;184:311–324. doi: 10.1016/S0079-6123(10)84016-6. [DOI] [PubMed] [Google Scholar]
- 7.McIntyre CC, Grill WM, Sherman DL, Thakor NV. Cellular effects of deep brain stimulation: model-based analysis of activation and inhibition. J Neurophysiol. 2004;91:1457–1469. doi: 10.1152/jn.00989.2003. [DOI] [PubMed] [Google Scholar]
- 8.Bar-Gad I, Elias S, Vaadia E, Bergman H. Complex locking rather than complete cessation of neuronal activity in the globus pallidus of a 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-treated primate in response to pallidal microstimulation. J Neurosci. 2004;24:7410–7419. doi: 10.1523/JNEUROSCI.1691-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nowak LG, Bullier J. Axons, but not cell bodies, are activated by electrical stimulation in cortical gray matter. I. Evidence from chronaxie measurements. Exp Brain Res. 1998;118:477–488. doi: 10.1007/s002210050304. [DOI] [PubMed] [Google Scholar]
- 10••.Gradinaru V, Mogri M, Thompson KR, Henderson JM, Deisseroth K. Optical deconstruction of parkinsonian neural circuitry. Science. 2009;324:354–359. doi: 10.1126/science.1167093. This rodent study investigated the cellular mechanisms of DBS for PD by attempting to replicate its effect through projection-specific optogenetic stimulation or inhibition, revealing that stimulation of afferent axons, not modulation of local cell bodies, might mediate the effects of DBS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hamani C, Diwan M, Macedo CE, Brandao ML, Shumake J, Gonzalez-Lima F, Raymond R, Lozano AM, Fletcher PJ, Nobrega JN. Antidepressant-like effects of medial prefrontal cortex deep brain stimulation in rats. Biol Psychiatry. 2010;67:117–124. doi: 10.1016/j.biopsych.2009.08.025. [DOI] [PubMed] [Google Scholar]
- 12.McConnell GC, So RQ, Hilliard JD, Lopomo P, Grill WM. Effective deep brain stimulation suppresses low-frequency network oscillations in the basal ganglia by regularizing neural firing patterns. J Neurosci. 2012;32:15657–15668. doi: 10.1523/JNEUROSCI.2824-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li Q, Ke Y, Chan DC, Qian ZM, Yung KK, Ko H, Arbuthnott GW, Yung WH. Therapeutic deep brain stimulation in Parkinsonian rats directly influences motor cortex. Neuron. 2012;76:1030–1041. doi: 10.1016/j.neuron.2012.09.032. [DOI] [PubMed] [Google Scholar]
- 14.Figee M, de Koning P, Klaassen S, Vulink N, Mantione M, van den Munckhof P, Schuurman R, van Wingen G, van Amelsvoort T, Booij J, et al. Deep brain stimulation induces striatal dopamine release in obsessive-compulsive disorder. Biol Psychiatry. 2014;75:647–652. doi: 10.1016/j.biopsych.2013.06.021. [DOI] [PubMed] [Google Scholar]
- 15.Halpern CH, Tekriwal A, Santollo J, Keating JG, Wolf JA, Daniels D, Bale TL. Amelioration of binge eating by nucleus accumbens shell deep brain stimulation in mice involves D2 receptor modulation. J Neurosci. 2013;33:7122–7129. doi: 10.1523/JNEUROSCI.3237-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hamani C, Machado DC, Hipolide DC, Dubiela FP, Suchecki D, Macedo CE, Tescarollo F, Martins U, Covolan L, Nobrega JN. Deep brain stimulation reverses anhedonic-like behavior in a chronic model of depression: role of serotonin and brain derived neurotrophic factor. Biol Psychiatry. 2012;71:30–35. doi: 10.1016/j.biopsych.2011.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17••.Bekar L, Libionka W, Tian GF, Xu Q, Torres A, Wang X, Lovatt D, Williams E, Takano T, Schnermann J, et al. Adenosine is crucial for deep brain stimulation-mediated attenuation of tremor. Nat Med. 2008;14:75–80. doi: 10.1038/nm1693. This rodent study demonstrated the importance of glia in the cellular mechanism of DBS. Adenosine, derived from ATP released from local astrocytes in response to DBS, depressed excitatory transmission in the thalamus and was necessary and sufficient to attenuate tremor. [DOI] [PubMed] [Google Scholar]
- 18.Etievant A, Lambas-Senas L, Scarna H, Lucas G, Haddjeri N. Astrocytes and gliotransmitters: new players in the treatment of major depression? Curr Drug Targets. 2013;14:1295–1307. doi: 10.2174/13894501113149990197. [DOI] [PubMed] [Google Scholar]
- 19••.Perez-Caballero L, Perez-Egea R, Romero-Grimaldi C, Puigdemont D, Molet J, Caso JR, Mico JA, Perez V, Leza JC, Berrocoso E. Early responses to deep brain stimulation in depression are modulated by anti-inflammatory drugs. Mol Psychiatry. 2014;19:607–614. doi: 10.1038/mp.2013.63. This study provided intriguing evidence in rodents and humans that the inflammatory response and reactive gliosis from electrode implantation could by itself have an antidepressant effect, prompting a re-evaluation of our understanding of the early effects of DBS, and supporting a role for glia in the mechanism of DBS for depression. [DOI] [PubMed] [Google Scholar]
- 20.Vedam-Mai V, van Battum EY, Kamphuis W, Feenstra MG, Denys D, Reynolds BA, Okun MS, Hol EM. Deep brain stimulation and the role of astrocytes. Mol Psychiatry. 2012;17:124–131. 115. doi: 10.1038/mp.2011.61. [DOI] [PubMed] [Google Scholar]
- 21.Ilse S, Pienaar CHL, Joanna L, Elson Louisa McGuinness, Gentleman Stephen M, Kalaria Raj N, Dexter David T. Deep-brain stimulation associates with improved microvascular integrity in the subthalamic nucleus in Parkinson’s disease. Neurobiol Dis. 2014 doi: 10.1016/j.nbd.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 22.van Hartevelt TJ, Cabral J, Deco G, Moller A, Green AL, Aziz TZ, Kringelbach ML. Neural plasticity in human brain connectivity: the effects of long term deep brain stimulation of the subthalamic nucleus in Parkinson’s disease. PLoS One. 2014;9:e86496. doi: 10.1371/journal.pone.0086496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nuttin B, Cosyns P, Demeulemeester H, Gybels J, Meyerson B. Electrical stimulation in anterior limbs of internal capsules in patients with obsessive-compulsive disorder. Lancet. 1999;354:1526. doi: 10.1016/S0140-6736(99)02376-4. [DOI] [PubMed] [Google Scholar]
- 24.Gillan CM, Papmeyer M, Morein-Zamir S, Sahakian BJ, Fineberg NA, Robbins TW, de Wit S. Disruption in the balance between goal-directed behavior and habit learning in obsessive-compulsive disorder. Am J Psychiatry. 2011;168:718–726. doi: 10.1176/appi.ajp.2011.10071062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Burguiere E, Monteiro P, Mallet L, Feng G, Graybiel AM. Striatal circuits, habits, and implications for obsessive-compulsive disorder. Curr Opin Neurobiol. 2015;30C:59–65. doi: 10.1016/j.conb.2014.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Greenberg BD, Gabriels LA, Malone DA, Jr, Rezai AR, Friehs GM, Okun MS, Shapira NA, Foote KD, Cosyns PR, Kubu CS, et al. Deep brain stimulation of the ventral internal capsule/ventral striatum for obsessive-compulsive disorder: worldwide experience. Mol Psychiatry. 2010;15:64–79. doi: 10.1038/mp.2008.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Denys D, Mantione M, Figee M, van den Munckhof P, Koerselman F, Westenberg H, Bosch A, Schuurman R. Deep brain stimulation of the nucleus accumbens for treatment-refractory obsessive-compulsive disorder. Arch Gen Psychiatry. 2010;67:1061–1068. doi: 10.1001/archgenpsychiatry.2010.122. [DOI] [PubMed] [Google Scholar]
- 28.Mallet L, Polosan M, Jaafari N, Baup N, Welter ML, Fontaine D, du Montcel ST, Yelnik J, Chereau I, Arbus C, et al. Subthalamic nucleus stimulation in severe obsessive-compulsive disorder. N Engl J Med. 2008;359:2121–2134. doi: 10.1056/NEJMoa0708514. [DOI] [PubMed] [Google Scholar]
- 29••.Hamani C, Temel Y. Deep brain stimulation for psychiatric disease: contributions and validity of animal models. Sci Transl Med. 2012;4:142rv148. doi: 10.1126/scitranslmed.3003722. This review summarizes DBS mechanisms in psychiatric disorders, with an emphasis on preclinical studies. [DOI] [PubMed] [Google Scholar]
- 30.Baas JM, Klumpers F, Mantione MH, Figee M, Vulink NC, Schuurman PR, Mazaheri A, Denys D. No impact of deep brain stimulation on fear-potentiated startle in obsessive-compulsive disorder. Front Behav Neurosci. 2014;8:305. doi: 10.3389/fnbeh.2014.00305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rodriguez-Romaguera J, Do Monte FH, Quirk GJ. Deep brain stimulation of the ventral striatum enhances extinction of conditioned fear. Proc Natl Acad Sci U S A. 2012;109:8764–8769. doi: 10.1073/pnas.1200782109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ahmari SE, Spellman T, Douglass NL, Kheirbek MA, Simpson HB, Deisseroth K, Gordon JA, Hen R. Repeated cortico-striatal stimulation generates persistent OCD-like behavior. Science. 2013;340:1234–1239. doi: 10.1126/science.1234733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Burguiere E, Monteiro P, Feng G, Graybiel AM. Optogenetic stimulation of lateral orbitofronto-striatal pathway suppresses compulsive behaviors. Science. 2013;340:1243–1246. doi: 10.1126/science.1232380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34••.Figee M, Luigjes J, Smolders R, Valencia-Alfonso CE, van Wingen G, de Kwaasteniet B, Mantione M, Ooms P, de Koning P, Vulink N, et al. Deep brain stimulation restores frontostriatal network activity in obsessive-compulsive disorder. Nat Neurosci. 2013;16:386–387. doi: 10.1038/nn.3344. This study provided direct evidence in OCD patients that NAc DBS reversed pathological frontostriatal hyperconnectivity in a manner which correlates with symptom reduction, and that it reduced pathological cortical low-frequency oscillations. [DOI] [PubMed] [Google Scholar]
- 35.Bourne SK, Eckhardt CA, Sheth SA, Eskandar EN. Mechanisms of deep brain stimulation for obsessive compulsive disorder: effects upon cells and circuits. Front Integr Neurosci. 2012;6:29. doi: 10.3389/fnint.2012.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Do-Monte FH, Rodriguez-Romaguera J, Rosas-Vidal LE, Quirk GJ. Deep brain stimulation of the ventral striatum increases BDNF in the fear extinction circuit. Front Behav Neurosci. 2013;7:102. doi: 10.3389/fnbeh.2013.00102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schmuckermair C, Gaburro S, Sah A, Landgraf R, Sartori SB, Singewald N. Behavioral and neurobiological effects of deep brain stimulation in a mouse model of high anxiety- and depression-like behavior. Neuropsychopharmacology. 2013;38:1234–1244. doi: 10.1038/npp.2013.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pierce RC, Vassoler FM. Deep brain stimulation for the treatment of addiction: basic and clinical studies and potential mechanisms of action. Psychopharmacology (Berl) 2013;229:487–491. doi: 10.1007/s00213-013-3214-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hamani C, McAndrews MP, Cohn M, Oh M, Zumsteg D, Shapiro CM, Wennberg RA, Lozano AM. Memory enhancement induced by hypothalamic/fornix deep brain stimulation. Ann Neurol. 2008;63:119–123. doi: 10.1002/ana.21295. [DOI] [PubMed] [Google Scholar]
- 40.Laxton AW, Tang-Wai DF, McAndrews MP, Zumsteg D, Wennberg R, Keren R, Wherrett J, Naglie G, Hamani C, Smith GS, et al. A phase I trial of deep brain stimulation of memory circuits in Alzheimer’s disease. Ann Neurol. 2010;68:521–534. doi: 10.1002/ana.22089. [DOI] [PubMed] [Google Scholar]
- 41.Suthana N, Fried I. Deep brain stimulation for enhancement of learning and memory. Neuroimage. 2014;85(Pt 3):996–1002. doi: 10.1016/j.neuroimage.2013.07.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stone SS, Teixeira CM, Devito LM, Zaslavsky K, Josselyn SA, Lozano AM, Frankland PW. Stimulation of entorhinal cortex promotes adult neurogenesis and facilitates spatial memory. J Neurosci. 2011;31:13469–13484. doi: 10.1523/JNEUROSCI.3100-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mayberg HS, Liotti M, Brannan SK, McGinnis S, Mahurin RK, Jerabek PA, Silva JA, Tekell JL, Martin CC, Lancaster JL, et al. Reciprocal limbic-cortical function and negative mood: converging PET findings in depression and normal sadness. Am J Psychiatry. 1999;156:675–682. doi: 10.1176/ajp.156.5.675. [DOI] [PubMed] [Google Scholar]
- 44.Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C, Schwalb JM, Kennedy SH. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45:651–660. doi: 10.1016/j.neuron.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 45.Morishita T, Fayad SM, Higuchi MA, Nestor KA, Foote KD. Deep brain stimulation for treatment-resistant depression: systematic review of clinical outcomes. Neurotherapeutics. 2014;11:475–484. doi: 10.1007/s13311-014-0282-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46••.Riva-Posse P, Choi KS, Holtzheimer PE, McIntyre CC, Gross RE, Chaturvedi A, Crowell AL, Garlow SJ, Rajendra JK, Mayberg HS. Defining critical white matter pathways mediating successful subcallosal cingulate deep brain stimulation for treatment-resistant depression. Biol Psychiatry. 2014;76:963–969. doi: 10.1016/j.biopsych.2014.03.029. This human study used probabilistic tractography to define the white matter pathways which define response to SCC DBS for depression, demonstrating an approach to improve DBS targeting and patient outcomes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dobrossy MD, Furlanetti LL, Coenen VA. Electrical stimulation of the medial forebrain bundle in pre-clinical studies of psychiatric disorders. Neurosci Biobehav Rev. 2014;49C:32–42. doi: 10.1016/j.neubiorev.2014.11.018. [DOI] [PubMed] [Google Scholar]
- 48.Crowell AL, Riva-Posse P, Garlow SJ, Mayberg HS. Toward an understanding of the neural circuitry of major depressive disorder through the clinical response to deep brain stimulation of different anatomical targets. Curr Behav Neurosci Rep. 2014;1:55–63. [Google Scholar]
- 49••.Veerakumar A, Challis C, Gupta P, Da J, Upadhyay A, Beck SG, Berton O. Antidepressant-like effects of cortical deep brain stimulation coincide with pro-neuroplastic adaptations of serotonin systems. Biol Psychiatry. 2014;76:203–212. doi: 10.1016/j.biopsych.2013.12.009. This rodent study showed that chronic vmPFC DBS reversed multiple forms of maladaptive plasticity in serotonergic networks in a mouse model of depression, indicating a possible mechanism for the long-term reinforcement of the antidepressant effects of SCC DBS in humans. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hamani C, Amorim BO, Wheeler AL, Diwan M, Driesslein K, Covolan L, Butson CR, Nobrega JN. Deep brain stimulation in rats: different targets induce similar antidepressant-like effects but influence different circuits. Neurobiol Dis. 2014;71:205–214. doi: 10.1016/j.nbd.2014.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lammel S, Tye KM, Warden MR. Progress in understanding mood disorders: optogenetic dissection of neural circuits. Genes Brain Behav. 2014;13:38–51. doi: 10.1111/gbb.12049. [DOI] [PubMed] [Google Scholar]
- 52.Qi J, Zhang S, Wang HL, Wang H, de Jesus Aceves Buendia J, Hoffman AF, Lupica CR, Seal RP, Morales M. A glutamatergic reward input from the dorsal raphe to ventral tegmental area dopamine neurons. Nat Commun. 2014;5:5390. doi: 10.1038/ncomms6390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shabel SJ, Proulx CD, Trias A, Murphy RT, Malinow R. Input to the lateral habenula from the basal ganglia is excitatory, aversive, and suppressed by serotonin. Neuron. 2012;74:475–481. doi: 10.1016/j.neuron.2012.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shabel SJ, Proulx CD, Piriz J, Malinow R. GABA/glutamate co-release controls habenula output and is modified by antidepressant treatment. Science. 2014;345:1494–1498. doi: 10.1126/science.1250469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55••.Li B, Piriz J, Mirrione M, Chung C, Proulx CD, Schulz D, Henn F, Malinow R. Synaptic potentiation onto habenula neurons in the learned helplessness model of depression. Nature. 2011;470:535–539. doi: 10.1038/nature09742. This rodent study showed that learned helplessness potentiates excitatory input onto VTA-projecting LHb neurons and that LHb DBS restores synaptic drive onto these LHb neurons by depleting excitatory synapses. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dzirasa K, Lisanby SH. How does deep brain stimulation work? Biol Psychiatry. 2012;72:892–894. doi: 10.1016/j.biopsych.2012.09.013. [DOI] [PubMed] [Google Scholar]
- 57.Srejic LR, Prescott IA, Zhang P, Strauss I, Dostrovsky JO, Giacobbe P, Kennedy SH, Lozano AM, Hamani C, Hutchison WD. Paired Pulse Depression in the Subcallosal Cingulate Region of Depression Patients. Biol Psychiatry. 2014 doi: 10.1016/j.biopsych.2014.09.018. In Press. [DOI] [PubMed] [Google Scholar]
- 58.Quraan MA, Protzner AB, Daskalakis ZJ, Giacobbe P, Tang CW, Kennedy SH, Lozano AM, McAndrews MP. EEG power asymmetry and functional connectivity as a marker of treatment effectiveness in DBS surgery for depression. Neuropsychopharmacology. 2014;39:1270–1281. doi: 10.1038/npp.2013.330. [DOI] [PMC free article] [PubMed] [Google Scholar]