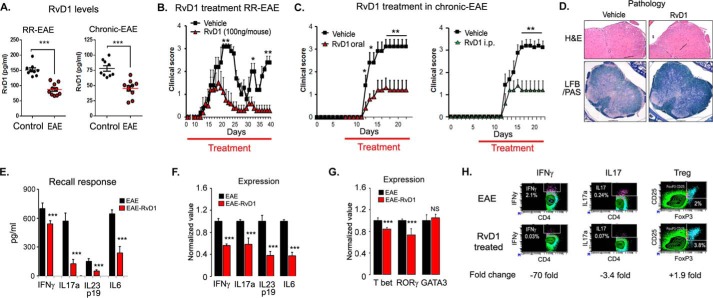
FIGURE 5.
Oral administration of RvD1 attenuates EAE disease progression. A, levels of RvD1 in plasma of RR- and chronic EAE by enzyme immunoassay (n = 9–10). B, SJL mice were immunized with PLP(139–151) on day 0 in complete Freund's adjuvant. One set of the group was given daily RvD1 from day 0 by gavage and another set was given vehicle in the same manner. Red line indicates the duration of treatment. Clinical score was taken until the end of the study (n = 10). C, B6 mice were immunized with MOG(35–55) on day 0 in complete Freund's adjuvant. One set of the group were given daily RvD1 from day 7 by gavage or intraperitoneally, and another set was given vehicle in the same manner. Clinical score was measured daily until end of the study (n = 8). Red line indicates the duration of treatment. D, photographs of spinal cord sections show inflammation (H&E; ×4 magnification) and demyelination (Luxol fast blue-periodic acid-Schiff; LFB/PAS, ×10 magnification) in both groups. Cells were stimulated with phorbol 12-myristate 13-acetate/ionomycin for 4 h in the presence of GolgiPlug. E, spleen cells were harvested and stimulated with PLP(139–151). Post-96 h, cell supernatants were used for ELISA (n = 4). F and G, for quantitative PCR, cells were harvested at 24 h post-stimulation with peptide, and expression of target genes was examined using quantitative PCR after normalizing with ribosomal protein L27 (n = 4). H, at 96 h of peptide stimulation, cells were stimulated with phorbol 12-myristate 13-acetate/ionomycin for 4 h in the presence of GolgiPlug. IL17a-, IFNγ-, and regulatory T cells (CD4+CD25+FoxP3)-expressing cells were measured by intracellular staining on a CD4+ gate. ***, p < 0.001; **, p < 0.01, and *, p < 0.05 compared with EAE.