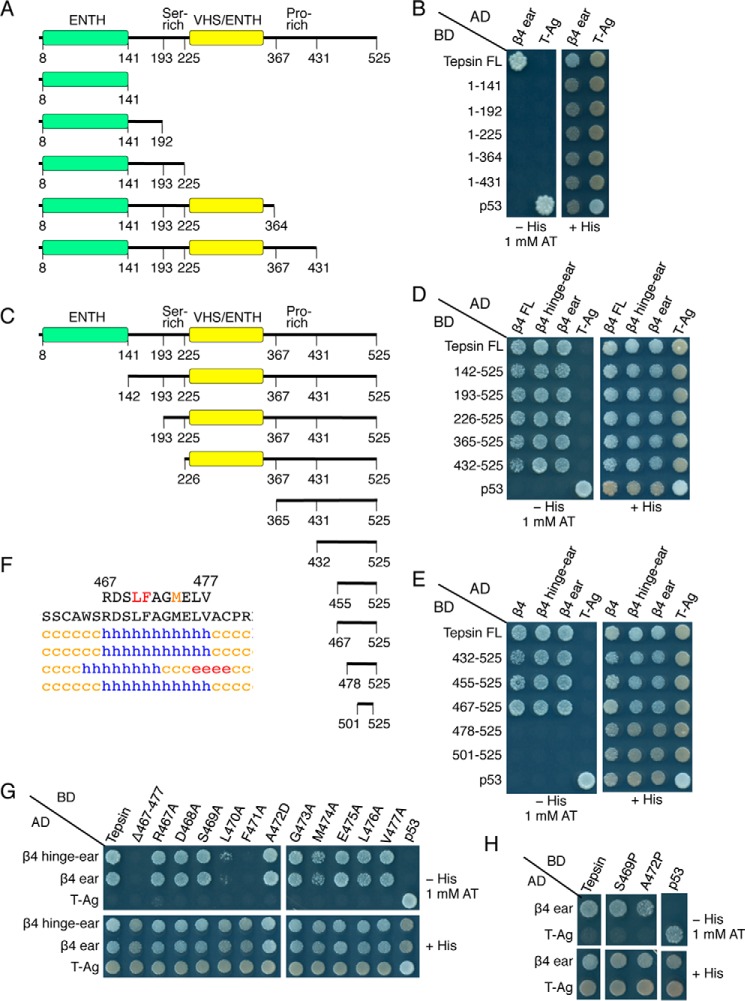
FIGURE 2.
Mutational and yeast two-hybrid analyses identify a short peptide sequence in tepsin that interacts with the AP-4 β4-ear domain. A, schematic representation of C-terminally truncated tepsin constructs, and B, testing for their interactions with the β4-ear domain using the Y2H system. C, schematic representation of N-terminally truncated tepsin constructs, and D and E, testing for their interactions with full-length (FL) β4, β4-hinge-ear and β4-ear constructs using the Y2H system. F, tepsin sequence required for interaction with the β4 ear, and secondary structure prediction by the methods described in the legend to Fig. 1B. Blue h represents α-helix, red e represents β-sheet, and orange c represents random coil structure. G and H, Y2H analysis of the interaction of tepsin mutants bearing single amino acid substitutions with the β4-hinge-ear and β4-ear domains. Y2H assays were performed as described in the legend to Fig. 1D.