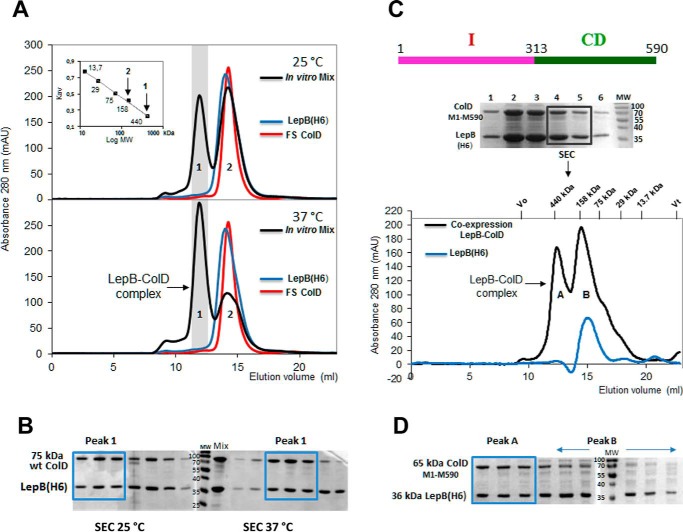
FIGURE 1.
Complex formation between LepB and colicin D in vitro and formation of the stable LepB-ColD complex after the co-expression of partners, characterized by SEC. A, SEC profiles of LepB(His6) (blue curve), FS colicin D (red curve), and an equimolar ratio of the two proteins mixed in vitro and incubated for 1 h at 25 °C (upper panel) or at 37 °C (bottom panel) are shown. The co-eluted LepB-FS ColD complex separated from both the free LepB and colicin D were solved as peaks 1 and 2 of the chromatogram (black curve), respectively. The Mr calibration curve was obtained with a series of commercial, soluble, standard proteins (ferritin 440 kDa, Stokes radius 61 Å; aldolase 158 kDa, Stokes radius 48.1 Å; conalbumin 75 kDa, Stokes radius 36.4 Å; carbonic anhydrase 29 kDa, Stokes radius 23 Å, and ribonuclease 13.7 kDa, Stokes radius 16.4 Å); for each the constant Kav is plotted against the log Mr (inset). Vertical arrows indicate the apparent Mr for the two peaks. mAU, milliabsorbance units. B, proteins of fractions collected during the SEC elution of the mixed complex, preincubated at 25 or 37 °C were analyzed by 15% SDS-PAGE. SEC fractions corresponding to the LepB-ColD complex (Peak 1) are boxed ahead of those of free partners (Peak 2). C, pLEPB1-wt expressing His-tagged LepB, carrying its intact transmembrane domains and pColD-E expressing C-terminally truncated colicin D (Met-1–Met-590) were co-expressed in BL21(DE3) cells (Table 1). The sonication of cells was followed by 1 h of incubation at 25 °C and then solubilized LepB was co-purified by Ni-NTA affinity chromatography with colicin D (Met-1–Met-590) as shown by SDS-PAGE. Two elution fractions together were subsequently analyzed by SEC and gave two peaks (black curve). The LepB-ColD (Met-1–Met-590) complex, eluted as peak A, had an apparent molecular mass of ∼430 kDa. Peak B was close to the single elution peak of the free monomeric LepB chromatogram (blue curve). The monomeric LepB with the micelle formed by the detergent Triton X-100 (90 kDa) had an apparent molecular mass of ∼145 kDa, as also shown in A. D, the SEC elution fractions were analyzed by SDS-PAGE. The stoichiometry of the LepB-ColD complex appeared to support a 1:1 molar ratio of the two partners (boxed fractions; see also B). The free LepB was enriched in peak B (indicated by a horizontal arrow).