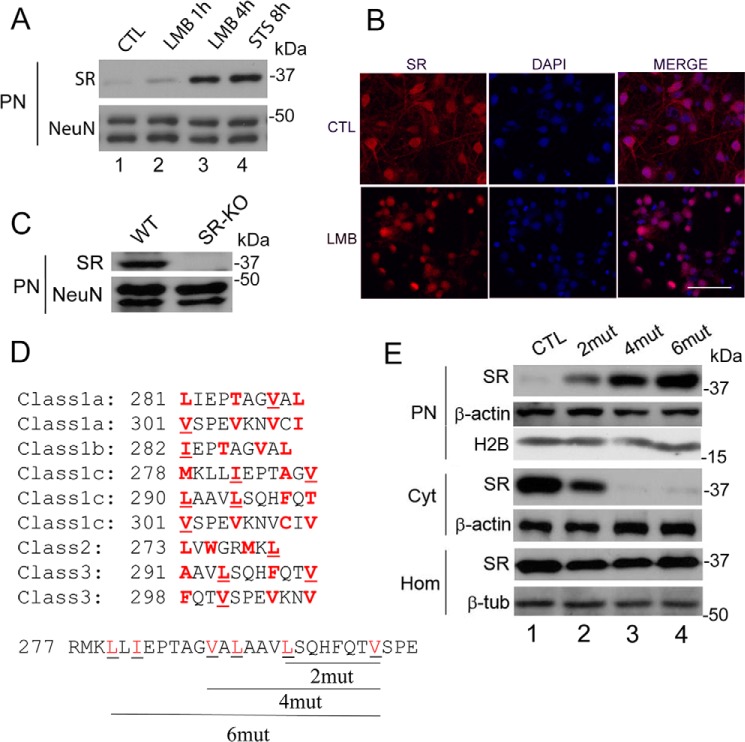
FIGURE 2.
SR nuclear export inhibition and identification of nuclear export signals at SR. A, primary neuronal cultures at DIV10–12 were treated with LMB (20 nm) for 1 or 4 h or with STS for 8 h. Nuclear SR and loading control were monitored by Western blot analysis in purified nuclear fraction (PN). B, primary neuronal cultures were treated with LMB (20 nm) for 4 h and analyzed by immunocytochemistry and confocal laser microscopy. Untreated cells (control (CTL), upper panels) and LMB-treated cells (lower panels) are shown. Scale bar = 50 μm. C, purified nuclei from cortical brain tissue of wild type (WT) mice contain SR. Nuclei purified from SR-KO mice did not exhibit immunoreactivity to SR. NeuN served as loading control. D, five classes of putative nuclear export signals (NESs) were identified in the C terminus of SR. The hydrophobic amino acids that are critical for the NESs are highlighted in red. The underlined amino acids were mutated to Gln (glutamine) and corresponded to Leu-280 and Ile-282 (2mut), Leu-280, Ile-282, Val-288, and Leu-290 (4mut) or Leu-280, Ile-282, Val-288, Leu-290, Leu-294, and Val-301 (6mut), which disrupted 3, 5, and 9 of the putative NESs, respectively. E, mutations in putative NESs promoted nuclear accumulation of the protein. SR mutants harboring the mutations described in D were transfected into Neuro-2a cells, and levels of SR in the nuclear fraction and cytosol were monitored by Western blot analysis 48 h after transfection. Mutations in SR NESs promoted nuclear SR accumulation (upper panel), associated with a reduction in cytosolic SR (middle panel). No significant changes in total SR levels in the homogenate were observed (lower panel). PN, purified nucleus; Cyt, cytosol; H2B, histone 2B; Hom, total cell homogenate. The panels represent at least three experiments with different preparations.