FIGURE 3.
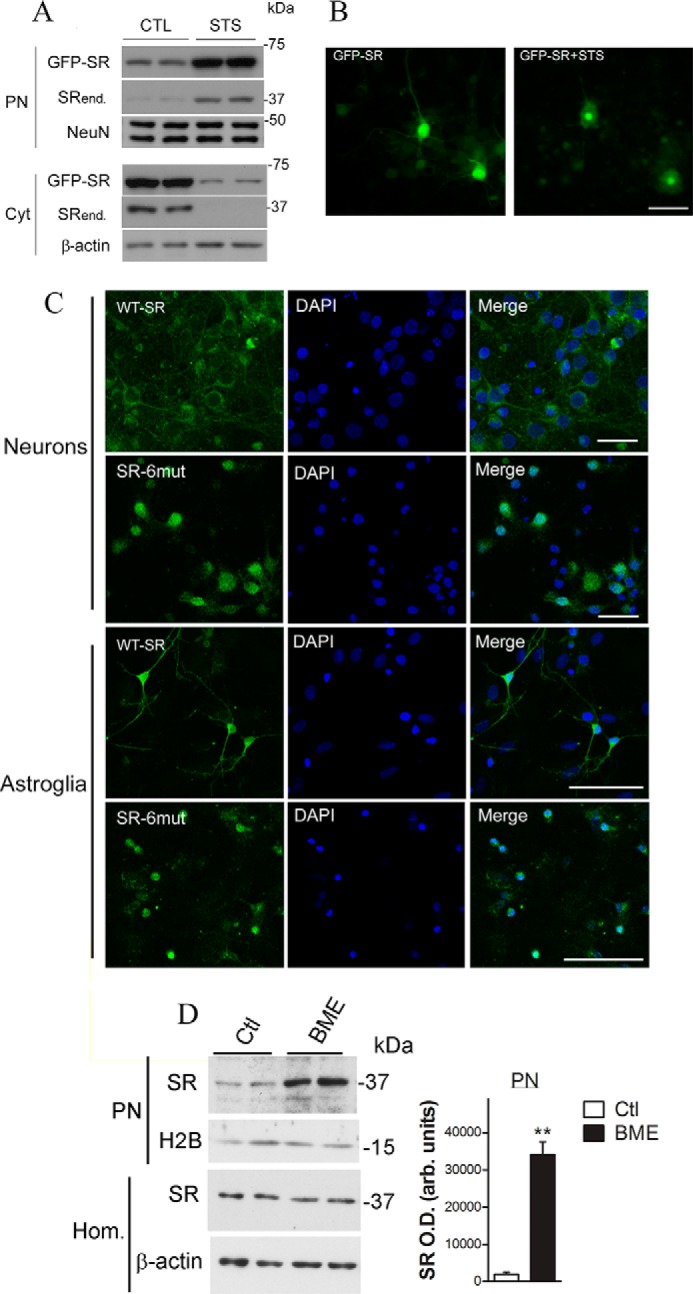
Lentivirus-infected primary neuronal cultures exhibited SR nuclear accumulation. A, primary neuronal cultures, DIV5, were infected with lentivirus harboring GFP-SR. On DIV10, neurons were treated with STS (1 μm) for 8 h. Nuclear (PN) and cytosolic (Cyt) fractions were monitored by Western blot analysis and probed with SR and loading controls. Translocation of GFP-SR to the nucleus took place simultaneously with a reduction in cytosolic levels. Endogenous SR (SRend.) also exhibited nuclear accumulation. B, live neurons (DIV10) were imaged every 10 min. After 30 min, STS (1 μm) was added to the medium, and the cells were further imaged for up to 24 h. GFP-SR was evenly distributed within the neuron (left panel), and complete translocation of GFP-SR to the nucleus was imaged 12 h after addition of STS (right panel). Scale bar, 50 μm. C, primary cortical neuronal cultures (DIV5) or primary astrocytes (DIV14) were incubated for 6 days with lentiviruses harboring GFP-SR WT (upper panels) or GFP-SR 6mut (lower panels). Both neurons and astrocytes expressing SR containing six NES mutations (SR-6mut) exhibited nuclear accumulation, as revealed by co-localization with DAPI and confocal laser microscopy. Scale bar, 35 μm. The panels represent at least three experiments with different preparations. D, BME treatment (12 h) promoted nuclear accumulation of SR and GAPDH in primary astrocyte cultures. Data represent average ± S.E. of four experiments with different astrocyte cultures. **, different from control at p < 0.001. Cyt, cytosolic fraction; H2B, histone H2B; Hom., homogenate; PN, purified nucleus.
