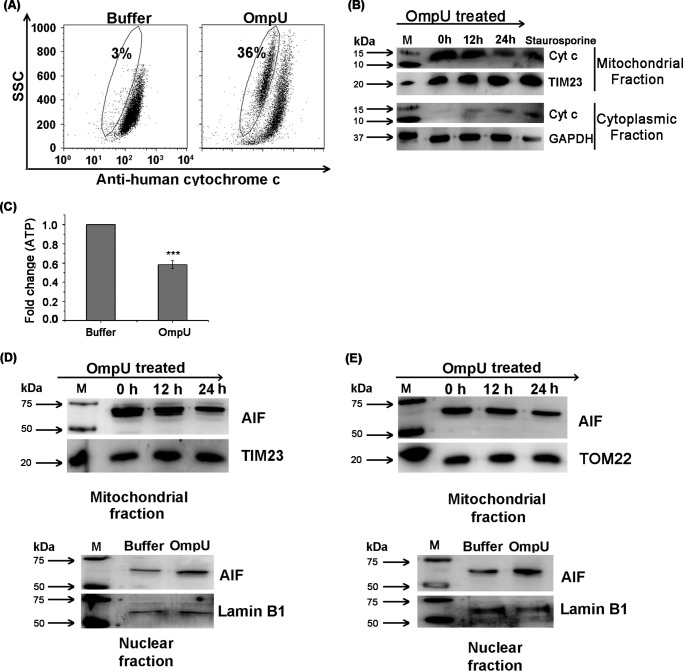
FIGURE 5.
OmpU induces cytochrome c and AIF release from mitochondria along with translocation of AIF to nucleus. A, dot plots showing considerable loss of cytochrome c in OmpU-treated cells compared with buffer. THP-1 monocytes were treated with 10 μg/ml OmpU or buffer and incubated for 24 h. Treated cells were harvested and stained with anti-human cytochrome c followed by FITC-conjugated anti-rabbit IgG. Decreased FITC fluorescence (gated population) corresponds to the decrease in cytochrome c in the mitochondria. Dot plots are representative of three independent experiments. B, Western blot showing release of cytochrome c from mitochondria to cytoplasm. THP-1 monocytes were treated with 10 μg/ml OmpU for different incubation periods and incubated for 0, 12, and 24 h. Cells were harvested, and their mitochondrial and cytoplasmic fractions were prepared, which were then analyzed for the release of cytochrome c by means of Western blot analysis. Blots are representative of three independent experiments. C, bar graph showing a reduction in the amount of ATP in OmpU-treated cells with respect to buffer-treated cells. THP-1 cells were treated with OmpU or buffer for 24 h and subjected to an ATP determination assay. The bar graph shows the mean ± S.E. (error bars) -fold change in the amount of ATP in OmpU-treated cells with respect to buffer calculated from three independent experiments. D and E, Western blot showing release of AIF from the mitochondrial fraction and its translocation to the nuclear fraction in OmpU-treated cells compared with control. THP-1 monocytes (D) and HEK 293 cells (E) were incubated with 10 μg/ml OmpU for 0, 12, and 24 h for analysis of their mitochondrial fractions and with OmpU or buffer for 24 h for analysis of their nuclear fractions. Mitochondrial and nuclear fractions were isolated from treated cells and analyzed for the presence of AIF by Western blotting. The blots are representative of three or more independent experiments.