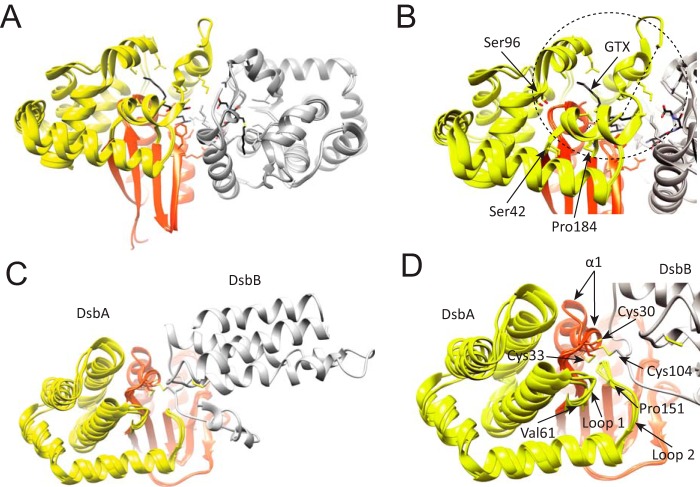
FIGURE 8.
Structural location of the α-helical subdomain in GST and DsbA. A, schematic representation of human κ class glutathione transferase (hGSTk). The transfer of GST reduced glutathione (GSH) to a variety of hydrophobic electrophiles for cellular detoxification. In this instance, the apo state of hGSTk (PDB code 3RPP) and when bound to a reaction product, S-hexylglutathione (GTX; PDB code 3RPN) is shown. B, close-up view of overlapping hGSTk structures where structural variability of the helical bundle can be observed; most evident is the variability within Ser42-Ser96 residues, which contains a region that structures upon substrate binding. C, schematic representation of disulfide bond oxidoreductases DsbA (orange-yellow) and DsbB (gray). DsbA is a periplasmic dithiol oxidase responsible for protein disulfide formation, DsbB is an integral membrane protein catalyzing the oxidation of DsbA by ubiquinone. Three crystal structures are overlapped, corresponding to the E. coli C33A DsbA mutant in complex with DsbB (PDB code 2HI7), oxidized DsbA (PDB code 1DSB), and C33A DsbA mutant (PDB code 1TI1). D. The canonical α1 of the thioredoxin-fold undergoes large conformational changes depending on its redox state. The helical bundle (yellow) shows significant structural variability between structures.