Table 1.
Condition Optimizationa.
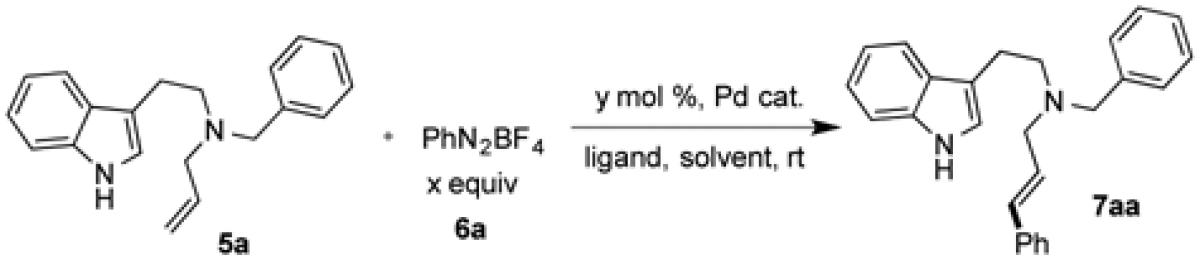
| |||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| entry | Pd cat. | x | y | solvent | ligand | base | yield (%)b |
|
| |||||||
| 1 | Pd(dba)2 | 1.5 | 5 | DMF | L1 | - | 54 |
| 2 | Pd(dba)2 | 1.5 | 5 | DMA | L1 | - | 33 |
| 3 | Pd(dba)2 | 1.5 | 5 | DMF | L1 | - | 49c |
| 4 | Pd(dba)2 | 1.5 | 5 | DMF | L1 | Et3N | - |
| 5 | Pd(dba)2 | 1.5 | 5 | DMF | L1 | K2CO3 | - |
| 6 | Pd(dba)2 | 2.0 | 5 | DMF | L1 | - | 73 |
| 7 | Pd(dba)2 | 2.5 | 5 | DMF | L1 | - | 71 |
| 8 | Pd(dba)2 | 2.0 | 8 | DMF | L1 | - | 80 |
| 9 | Pd(dba)2 | 2.0 | 10 | DMF | L1 | - | 85 |
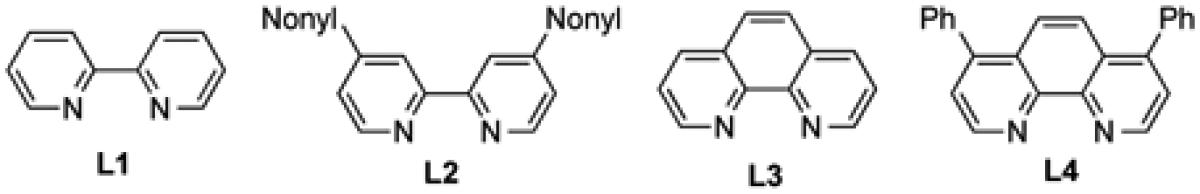
| 10 | Pd(dba)2 | 2.0 | 10 | DMF | L2 | - | 81 |
| 11 | Pd(dba)2 | 2.0 | 10 | DMF | L3 | - | 54 |
| 12 | Pd(dba)2 | 2.0 | 10 | DMF | L4 | - | 90 |
| 13 | Pd(dba)2 | 2.0 | 10 | DMF | - | - | 54 |
| 14 | Pd(OAc)2 | 2.0 | 10 | DMF | L4 | - | 55 |
| 15 | PdCl2 | 2.0 | 10 | DMF | L4 | - | trace |
| 16 | Pd(PPh3)4 | 2.0 | 10 | DMF | L4 | - | trace |
| |||||||
General reaction conditions unless otherwise noted: A mixture of palladium catalyst and ligand in DMF was stirred at rt for 20 min under argon before 5a and 6a were added. The reaction mixture was stirred at rt and monitored by thin-layer chromatography.
Isolated reaction yield.
60°C.
