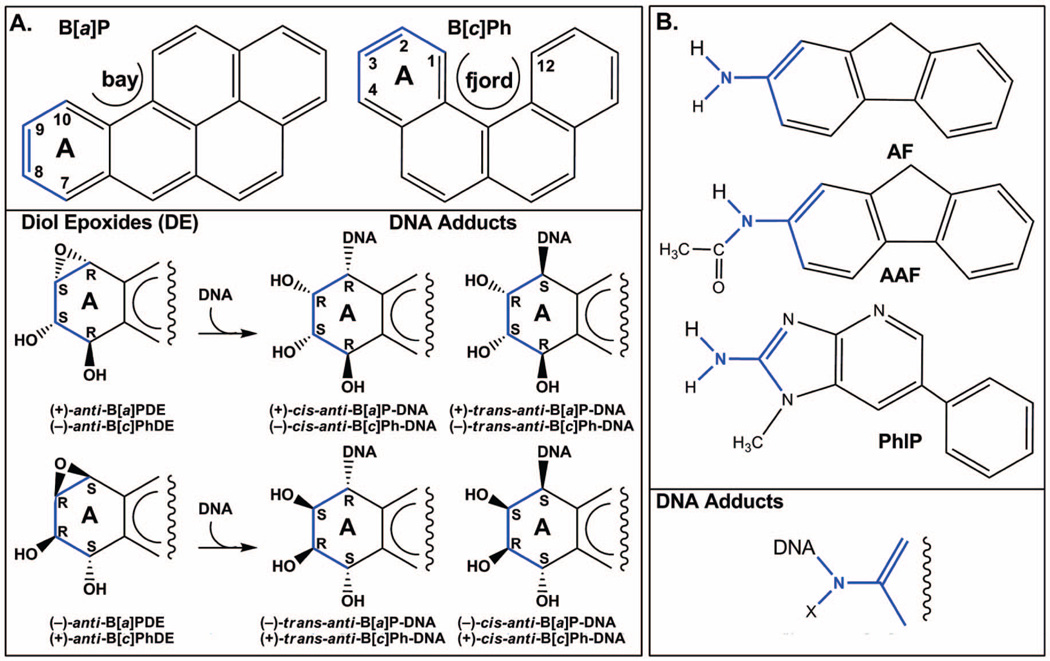
Figure 1.
Structures of representative PAHs (A) and polycyclic aromatic amines (B), and their DNA adducts. PhIP is a heterocyclic aromatic amine. PAHs are adducted to DNA via the N2 of guanine or the N6 of adenine. Polycyclic aromatic amines are adducted to DNA via the C8 of G. Chiral centers in the A ring of the PAH adducts and the linkage site of the polycyclic aromatic amine adducts are shown in blue. X is H or COCH3.