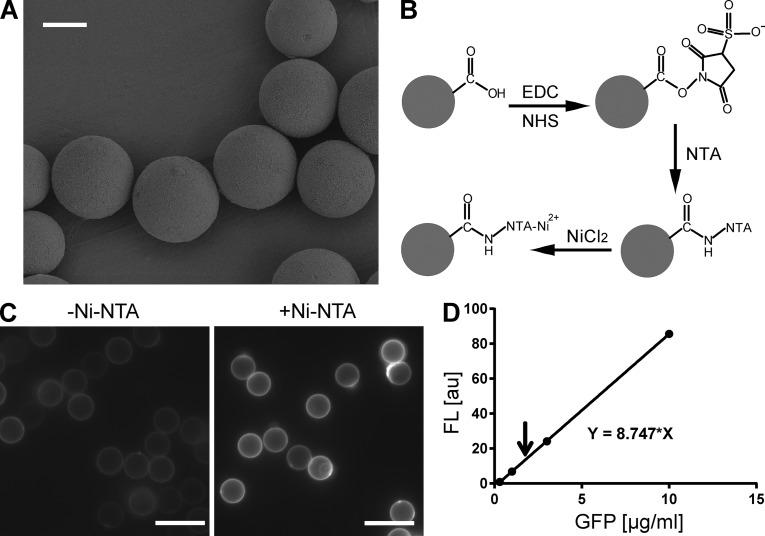
Figure 2.
Functionalization of silica beads with Ni-NTA groups. (A) Scanning electron microscopic (SEM) images of 10-µm micrometer beads. Bar, 5 µm. (B) Diagram of chemical functionalization on the surface of an oxidized silica bead. Only one carboxylate is drawn for simplicity. EDC/NHS was used to accelerate conjugation reaction and maximize reaction efficiency. (C) Fluorescent images of functionalized beads labeled with GFP-His6 proteins. Beads that were functionalized in the absence (left) or presence (right) of AB-NTA were compared. The latter showed significant labeling (right), suggesting a high density of Ni-NTA groups on the surface. Bars, 10 µm. (D) Estimation of the binding capacity of the surface Ni-NTA groups. A standard curve of fluorescence intensity was first prepared (dots and the continuous line). 0.10 mg GFP-His6 was incubated for 2 h with 10 mg Ni-NTA silica beads in 0.40 ml buffer containing 10 mM HEPES/KOH, pH 7.4, and 200 mM KCl. After the removal of unbound GFP-His6, the bound GFP-His6 was eluted with 0.30 M imidazole, and its fluorescence intensity (the arrow) was measured and compared with the standard curve. The measured signal corresponded to ∼0.84 µg GFP-His6 for 10-mg beads, which is ∼19 trillion GFP molecules. Based on the manufacturer’s information, the total surface area of the 10-mg beads is 5.8 × 109 µm2. The estimated binding capacity of the Ni-NTA beads is ∼3,300/µm2.