Figure 4.
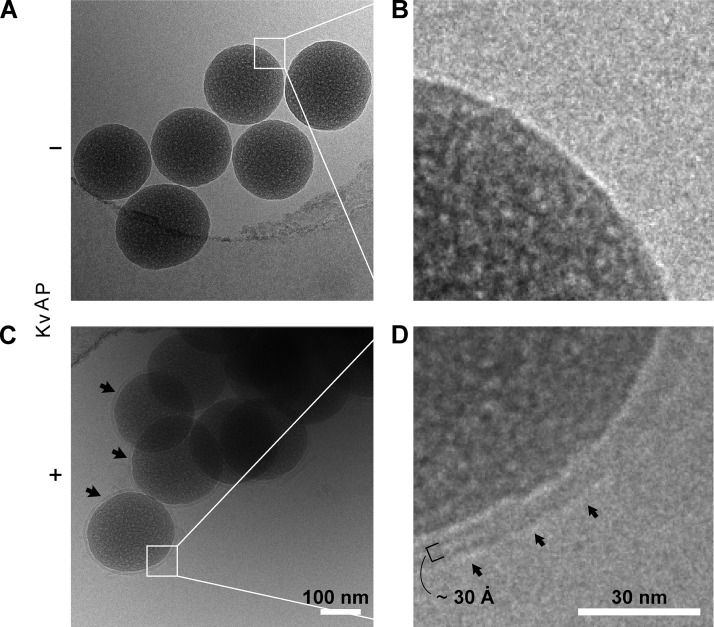
Unilamellar nature of bSUMs revealed by cryoEM. Beads of 0.20 µm in diameter were used to prepare the specimens. A PE/PG lipid mixture was used for membrane reconstitution. Beads prepared in the absence (A and B) or presence (C and D) of KvAP protein were compared under cryoEM. Multiple beads trapped close to the edge of a hole in a holey carbon–coated Quantifoil grid are shown in each image (A vs. C). The black arrows in C point to membranes around individual beads. B and D represent magnified views of the two areas marked with white squares in A and C, respectively. There is no clear membrane at the bead surface in B, but a characteristic bilayer structure (after convolution of the contrast transfer function of the EM) is seen in D. In D, the arrows mark the outer surface of the bSUM. The small black bracket, covering two dense bands and one light band, represents the thickness of the hydrophobic core of a typical bilayer, ∼3 nm.