Figure 5.
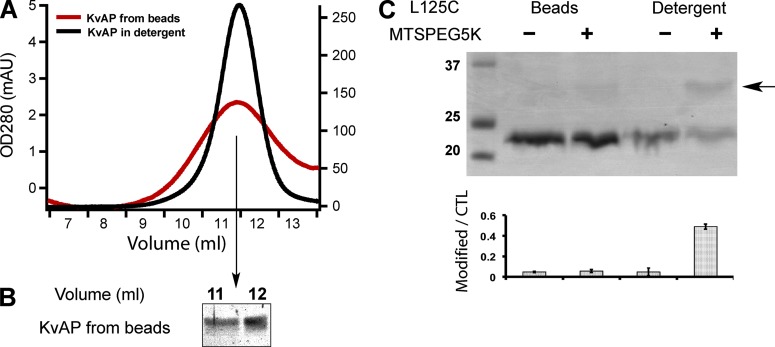
Unidirectional insertion of tetrameric Kv channels in bSUMs. (A) KvAP from bSUMs remains tetrameric. KvAP was extracted from the bSUMs by 40 mM DM, concentrated, and injected into a Superdex 200 size-exclusion column. The channels were eluted (red line) at the same position as those purified in detergents (black line). The slightly broader peak width could be caused by the lipids. (B) KvAP from the elution peak in A was collected and assayed via Coomassie blue–stained reducing SDS-PAGE. The channel protein showed no detectable degradation. (C) Inaccessibility of KvAP L125C in the bSUMs made of PE/PG lipids. KvAP in bSUMs or extracted in 40 mM DM were treated with MTS-PEG5K before being assayed by Coomassie blue–stained nonreducing SDS-PAGE. PEG5K conjugation shifted the channel band by ∼5 kD (arrow to the right side). The density ratio of the conjugated protein versus the unconjugated control (CTL) was plotted underneath the gel (bottom). The errors bars showed the range of variation from duplicate measurements. This experiment was repeated more than four times by different authors.