Abstract
Various scaffolds used in tissue engineering require a controlled biochemical environment to mimic the physiological cell niche. Interfacial polyelectrolyte complexation (IPC) fibers can be used for controlled delivery of various biological agents such as small molecule drugs, cells, proteins and growth factors. The simplicity of the methodology in making IPC fibers gives flexibility in its application for controlled biomolecule delivery. Here, we describe a method of incorporating IPC fibers into two different polymeric scaffolds, hydrophilic polysaccharide and hydrophobic polycaprolactone, to create a multi-component composite scaffold. We showed that IPC fibers can be easily embedded into these polymeric structures, enhancing the capability for sustained release and improved preservation of biomolecules. We also created a composite polymeric scaffold with topographical cues and sustained biochemical release that can have synergistic effects on cell behavior. Composite polymeric scaffolds with IPC fibers represent a novel and simple method of recreating the cellular niche.
Keywords: Bioengineering, Issue 102, composite scaffold, polymer, hydrogel, biochemicals, encapsulation, temporal, spatial, sustained release, topography
Introduction
The extracellular matrix has inherent biochemical and biophysical cues that direct cell behaviors. Mimicking this physiological three-dimensional (3D) microenvironment is a widely explored strategy for regenerative medicine and tissue engineering applications. For example, both naturally-derived and synthetic substrates have been modified with topographical cues as a means to mimic the biophysical cellular environment.1 For example, polycaprolactone (PCL) scaffolds can be easily patterned by casting on patterned PDMS substrates.2 However, most synthetic scaffolds inadequately recapitulate the controlled biochemical environment in vivo. Bulk or surface modification of synthetic materials only present biochemical cues for cell attachment but still lack temporal regulation of biochemical delivery.3 Thus, there is a need for optimal scaffolds that can mimic the temporally regulated biochemical delivery system of the extracellular matrix.
Biochemical delivery systems such as microspheres are plagued by problems of loss of bioactivity and low incorporation efficiency due to the severity and complexity of multi-step synthesis process.4-6 Alternative methods that use a one-step fabrication and incorporation method were proven to have excellent potential to create a favorable biochemical microenvironment without the accompanying inefficiency in incorporation and loss of bioactivity. One viable solution is the use of interfacial polyelectrolyte complexation (IPC) fibers to deliver and protect biological agents. When two oppositely charged polyelectrolyte aqueous solutions are brought together, IPC fibers can be drawn out from the interface. Virtually any type of hydrophlic biomolecule in aqueous solution can be added into either the negatively- or positively-charged polyelectrolyte solution, thus facilitating the incorporation of useful biomolecules into the IPC fiber during the complexation process. Furthermore, this process only requires aqueous and ambient conditions, thereby decreasing the risk of loss of bioactivity. Using this method, active growth factors2,7 even cells8,9 have been successfully delivered. In addition, the simple method of forming IPC fibers allows molding into any shape or orientation. The stability of such fibers has been advantageous in its incorporation into both hydrophobic2 and hydrophilic polymers7 to create composite scaffolds. These composite scaffolds with IPC fibers are beneficial for creating a physiologically relevant biochemical environment while providing physical anchorage for cells.
In this study, we show a method to incorporate IPC fibers into a hydrophilic and a hydrophobic scaffold with topography for controlled release of active biomolecules. As a proof-of-concept, we incorporate IPC fibers made from chitosan and alginate into the biocompatible, non-immunogenic and non-antigenic pullulan-dextran hydrophilic hydrogel or the biocompatible polycaprolactone hydrophobic scaffold.
Protocol
1. Preparation of Polyelectrolyte Solutions
Purify chitosan, as detailed in Liao et al. Briefly, create a 1% (w/v) solution of chitosan in 2% (v/v) acetic acid and vacuum filter using grade 93 filter paper. Neutralize the filtrate using 5M NaOH until the pH stabilized to 7. Centrifuge the precipitated chitosan at 1,200 x g for 10 min. Decant the supernatant and add deionized water to wash the chitosan. Repeat the centrifugation and washing step two more times. Freeze the precipitated chitosan at -80 °C and lyophilize O/N to obtain the purified form. Store purified chitosan in a dehumidified cabinet.
Weigh out 1 g of purified chitosan into a sterile tissue culture dish. Place the chitosan in the tissue culture dish as close as possible to the UV lamp in the biological safety cabinet and expose to UV light for 15 min. Using sterile forceps, place the sterilized chitosan into a glass container. Dissolve chitosan using filtered 0.15M acetic acid to a final concentration between 0.5% and 1% (w/v).
Weigh out 0.1 g of alginic acid sodium salt and dissolve in 10 ml distilled deionized (DDI) water to obtain a 1% (w/v) solution. Mix the alginic acid sodium salt for at least 2 hr on the vortex mixer to ensure complete dissolution. Filter the alginate solution through 0.2 µm syringe filter. Store the alginate solution at 4 °C.
Reconstitute human recombinant growth factors such as vascular endothelial growth factor (VEGF) or beta - nerve growth factor (NGF), as recommended by manufacturer.
2. Drawing of IPC Fibers
Mix proteins, growth factors or other biomolecules into 10-20 µl aliquot of the polyelectrolyte solution that has a similar net charge. Biological molecules with net negative charge (eg bovine serum albumin [BSA]) should be mixed with alginate solution. Biological molecules with net positive charge (eg VEGF) should be mixed with chitosan solution.
Place small aliquots (10-20 µl) of chitosan and alginate on a stable flat surface that is covered with parafilm. The droplets of chitosan and alginate should be placed in close proximity but not in contact with each other.
Lightly dip each tip on a pair of forceps into the chitosan and alginate droplets. Bring the droplets of polyelectrolytes together by pinching the forceps. When the droplets come into contact with each other, slowly pull the forceps vertically upward to draw the IPC fiber from the interface of the two droplets (Figure 1A).
Carefully place the end of the drawn IPC fiber on the forceps on a collector, such as a flat polymeric scaffold affixed on a rotating mandrel (see section 3 and 4). Rotate the mandrel at a fixed speed of 10 mm/sec to allow formation of uniform and beadless IPC fibers. Increasing the speed of drawing the IPC fibers will form beads, which will cause a burst release of incorporated biochemical and premature fiber termination.10
To determine incorporation efficiency, collect all the remaining liquids left on the parafilm by diluting with 500 µl of 1X phosphate buffered saline (PBS). Measure the protein or growth factor content in the residue through BCA assay (for BSA), ELISA (for VEGF and NGF) or an appropriate assay to detect incorporated biomolecule.
3. Fabrication of Composite Hydrogel Scaffold of Pullulan-Dextran (PD) Polysaccharide and IPC Fibers
- Fabricating sacrificial pullulan frame for IPC fiber collection
- Weigh out pullulan polysaccharide and mix with distilled deionized (DDI) water to create a 20% (w/v) aqueous solution. Mix the pullulan solution O/N to ensure homogeneity.
- Cast 15 g of pullulan solution into a 10 cm diameter tissue culture polystyrene (TCPS) dish. Dry the pullulan solution O/N at 37 °C. Cut the pullulan films into 7 mm x 7 mm square frames.
- Preparing pullulan-dextran polysaccharide solution
- Create a 30% (w/v) solution of the polysaccharides pullulan and dextran (3:1 ratio) in DDI water. Mix O/N to ensure homogeneity of the polysaccharide solution. Slowly add in sodium bicarbonate to the polysaccharide solution to achieve a final concentration of 20% (w/v). Mix O/N to ensure homogeneity of the solution. Store the polysaccharide solution at 4 °C.
- Collecting IPC fibers on pullulan frame
- Affix the sacrificial pullulan frame (section 3.1) using an alligator clip. Stick the alligator clip and pullulan frame on the end of the rotating mandrel using plastic-coated adhesive tape. Rotate the mandrel with the affixed frame at a constant speed of 10 mm/sec. The pullulan frame can be affixed onto the rotating mandrel in desired orientations.
Draw the IPC fibers using a pair of forceps (section 1) and attach the drawn end of the IPC fibers onto the rotating pullulan frame. Draw the IPC fibers at a constant speed. Upon reaching the terminal end of the IPC fiber, dry the fibers-on-frame construct O/N at RT.
Embedding IPC fibers into PD hydrogel scaffold
To crosslink every gram of pullulan-dextran solution, add 100 µl of 11% (w/v) sodium trimetaphosphate aqueous solution and 100 µl 10M sodium hydroxide.7 Mix the solution at 60 rpm using a stirplate for 1 to 2 min. After the addition of sodium trimetaphosphate and sodium hydroxide, the polysaccharide solution will crosslink almost immediately. Pour the viscous polysaccharide solution onto the fibers-on-frame construct to fully embed the IPC fibers. Incubate the combined pullulan-dextran-IPC fibers (PD-IPC) at 60 °C for 30 min to form a chemically crosslinked composite scaffolds (Figure 1B).
CAUTION: Perform step 3.3.2 in the fume hood and use proper protective equipment as acetic acid is a corrosive and flammable.
To induce pore formation in the PD-IPC scaffold, submerge the whole scaffold in 20% (w/v) acetic acid for 20 min.
Remove unreacted reagents by washing PD-IPC scaffolds in 1X PBS for 5 min while shaking at 100 rpm. Repeat this step 2 times.
Remove the excess PBS and immediately freeze the PD-IPC scaffolds at -80 °C O/N. Lyophilize the scaffolds at least 24 hr before use in any controlled release or bioactivity assays.
4. Fabrication of Composite Scaffold of PCL and IPC Fibers
CAUTION: Dichloromethane is a hazardous material. Use the fume hood and personal protective equipment when handling dichloromethane.
- Creating pristine and patterned PDMS substrates
- Create a pristine polydimethylsiloxane (PDMS) elastomeric substrate using a piece of TCPS of desired dimension using soft lithography process. Create patterned PDMS substrates by using standard soft lithographic methods on poly(methyl methacrylate) templates with the desired topography.12
- Fabricating sacrificial PCL frame for IPC fiber collection
- Weigh out PCL and dissolve in dichloromethane to create a 0.9% (w/v) solution. For every 1 cm2 area of the PDMS substrate, drop 500 µl of 0.9% PCL solution. Allow all of the dichloromethane solvent to fully evaporate in the fume hood. Repeat the process of casting 0.9% PCL to thicken the film to the desired thickness. Remove the dried PCL film from the PDMS substrate. Create a hole in the PCL frame using a suitably-sized puncher.2
- Collecting IPC fibers on the PCL frame
- Affix the sacrificial PCL frame with hole (from 4.2.1) on an alligator clip. Stick the alligator clip onto the rotating mandrel by using plastic-coated adhesive tape. Attach the drawn end of the IPC fiber onto the PCL frame before starting the rotation at a constant speed of 10mm/sec (section 2). After the end of IPC fiber drawing, dry the fiber-on-frame construct O/N at 4 °C.
- Embedding fiber-on-frame construct into patterned PCL substrate
- Drop 500 µl of 0.9% PCL solution onto the PDMS substrate to create a pristine or patterned PCL base, as required. Cast multiple layers of 0.9% PCL solution to obtain a scaffold with the desired thickness. Allow all of the dichloromethane solvent to fully evaporate in the fume hood.
- Place the fiber-on-frame construct (section 4.3.1) on top of the PCL base. Add 0.9% PCL solution on the fiber-on-frame construct multiple times to get the desired thickness and fully embed the IPC fibers, fabricating a PCL-IPC composite scaffold (Figure 1C).
5. Measurement of Release of Biological Agents from Composite IPC Scaffolds
Place composite PD-IPC or PCL-IPC scaffolds and stand-alone IPC fibers separately in a 24-well plate.
Immerse the scaffold and stand-alone IPC fibers with 500 µl of 1X PBS. Incubate the samples at 37 °C. Collect PBS at various time points (release media) and replace with 500 µl of 1X PBS.
Measure the amount of protein or growth factor in the release media using a BCA assay (BSA), ELISA (VEGF and NGF) or other appropriate assay to calculate the cumulative release profile for the incorporated biomolecule.
6. Seeding of Cells on Composite IPC Scaffolds to Test Bioactivity of Released Biological Agents
Sterilize the lyophilized PD-IPC or PCL-IPC composite scaffolds using UV light in the biological safety cabinet for at least 20 min.
Use standard cell culture techniques to obtain a cell suspension of 2 x 105 cells in 200 µl growth media. Seed the concentrated cell suspension onto the composite scaffolds. After 20 min, top-up the volume of growth media to fully submerge the scaffolds.
Measure cell activity through standard techniques such as Alamar blue metabolic activity assay, PC12 neurite outgrowth assay or immunofluorescence.
Representative Results
In this article, we sought to create composite scaffolds with IPC fibers for the sustained release of various biomolecules. Characteristics of the biomolecules used in this study are found in Table 1. IPC fibers were first embedded into a hydrophilic PD hydrogel to create a PD-IPC composite scaffold (Figure 1B). Model molecule BSA was first tested to determine the feasibility of using a composite scaffold for controlled biomolecule release. BSA was incorporated into PD-IPC scaffolds with an efficiency of 45 ± 0.97%. BSA released from the PD-IPD showed near-linear kinetics with an initial attenuated burst release followed by a concomitant steady state (Figure 2). After 2 months, BSA achieved a total release of 97%. In contrast, standalone IPC fibers exhibited a rapid release of 80% of BSA within 4 hr.
Next, we checked the release profile and bioactivity of VEGF using PD-IPC scaffolds. VEGF was incorporated with an efficiency of 75.5 ± 2.7%, and showed a sustained release for at least 1 week (Figure 3A). Human umbilical vein endothelial cells (HUVECs) were seeded on the PD-IPC scaffolds to determine the bioactivity of VEGF. HUVECs on the PD-IPC scaffolds showed a significant increase in Alamar blue reduction and metabolic activity compared with plain PD scaffolds at day 1, indicating good preservation of VEGF function after being released from PD-IPC scaffolds (Figure 3B). Alamar blue reduction at days 3, 6 and 7 decreased to achieve comparable levels with the plain PD scaffold (Figure 3B).
The PCL-IPC composite scaffolds were also tested for controlled release and compared with standalone IPC fibers. We incorporated NGF as a representative molecule into the PCL-IPC composite scaffolds with an incorporation efficiency of 66.38 ± 2.71%. PCL-IPC composite scaffolds showed linear sustained release and approximately 80% cumulative release after 18 days (Figure 4A). On the other hand, IPC stand-alone fiber showed a 70% burst release within 24 hr followed by a stagnant release rate. Using a PC12 neurite outgrowth assay, we examined the bioactivity of the released NGF (Figure 4B). The neurite outgrowth of PC12 cells grown on PCL-IPC composite scaffold release media showed similar levels with PC12 cells cultured in 30 ng/ml NGF supplemented media. This indicates that the released NGF remained bioactive for at least 7 days.
Combination of topography and sustained growth factor delivery may mimic the cellular microenvironment better. The versatile methodology of PCL-IPC fabrication allowed the fabrication of a biochemically- and topographically-controlled composite scaffold. We fabricated a PCL-IPC composite scaffold with nano-sized gratings structure (NP-PCL-IPC scaffold). We observed the synergistic effect of topography and sustained NGF release by assessing neuronal differentiation of human mesenchymal stem cells (hMSCs) (Figure 5). hMSCs cultured on the NP-PCL-IPC composite scaffolds showed higher expression of the Microtubule associated protein 2 (MAP2), indicative of neuronal differentiation. On the other hand, MAP2 protein expression was substantially lower in hMSCs cultured on PCL-IPC with only NGF release or patterned PCL (NP-PCL).
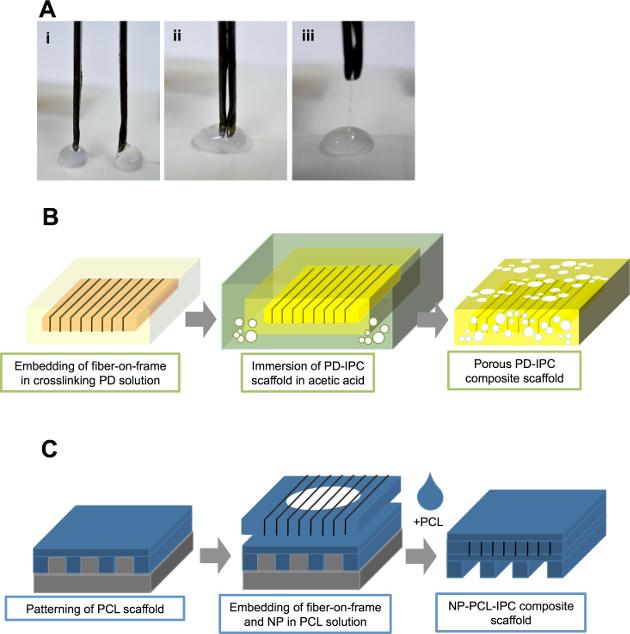 Figure 1. Incorporation of IPC fibers into hydrophilic and hydrophobic scaffolds. (A) Drawing of IPC fibers at the interface of positively (chitosan) and negatively (alginate) charged polyelectrolyte solutions. (B) Schematic diagram showing incorporation of IPC fibers in hydrophilic PD solution to create PD-IPC composite scaffold. (C) Schematic diagram showing incorporation of IPC fibers in hydrophobic PCL scaffold to create PCL-IPC composite scaffold. This figure is adapted from Cutiongco et al., 2014.7Please click here to view a larger version of this figure.
Figure 1. Incorporation of IPC fibers into hydrophilic and hydrophobic scaffolds. (A) Drawing of IPC fibers at the interface of positively (chitosan) and negatively (alginate) charged polyelectrolyte solutions. (B) Schematic diagram showing incorporation of IPC fibers in hydrophilic PD solution to create PD-IPC composite scaffold. (C) Schematic diagram showing incorporation of IPC fibers in hydrophobic PCL scaffold to create PCL-IPC composite scaffold. This figure is adapted from Cutiongco et al., 2014.7Please click here to view a larger version of this figure.
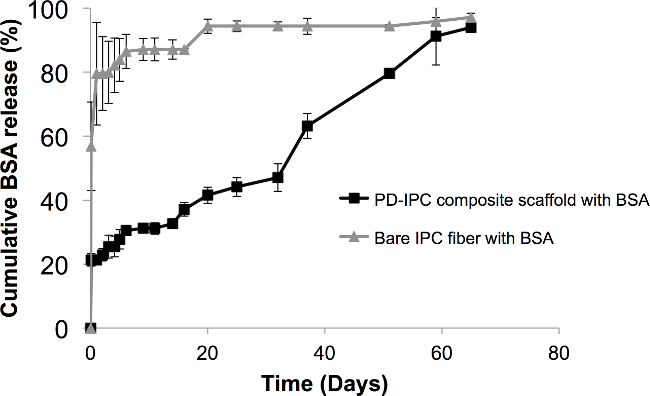 Figure 2. Controlled release of BSA from PD-IPC composite scaffold. BSA was incorporated into PD-IPC composite scaffolds and its release was measured at various time points using BCA assay. Cumulative BSA released is provided as a percentage of the total amount of BSA (in µg) incorporated in the IPC fibers and are presented as mean percentage ± standard deviation. This figure is adapted from Cutiongco et al., 2014.7
Please click here to view a larger version of this figure.
Figure 2. Controlled release of BSA from PD-IPC composite scaffold. BSA was incorporated into PD-IPC composite scaffolds and its release was measured at various time points using BCA assay. Cumulative BSA released is provided as a percentage of the total amount of BSA (in µg) incorporated in the IPC fibers and are presented as mean percentage ± standard deviation. This figure is adapted from Cutiongco et al., 2014.7
Please click here to view a larger version of this figure.
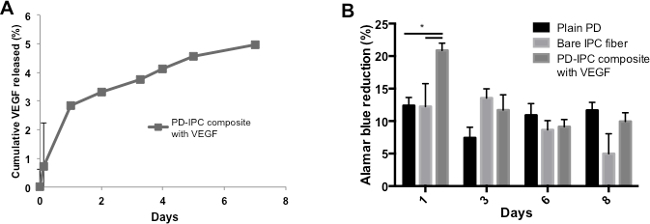 Figure 3. Controlled release and bioactivity of VEGF from PD-IPC composite scaffold. (A) Cumulative release profile of VEGF from PD-IPC composite scaffolds. VEGF release was measured at various time points using ELISA specific for VEGF. (B) Cell viability of endothelial cells grown on PD-IPC composite scaffolds, as measured by Alamar blue metabolic assay. Statistical analysis was performed using one-way ANOVA with Tukey’s post-hoc test. *denotes p < 0.05. This figure is adapted from Cutiongco et al., 2014.7
Please click here to view a larger version of this figure.
Figure 3. Controlled release and bioactivity of VEGF from PD-IPC composite scaffold. (A) Cumulative release profile of VEGF from PD-IPC composite scaffolds. VEGF release was measured at various time points using ELISA specific for VEGF. (B) Cell viability of endothelial cells grown on PD-IPC composite scaffolds, as measured by Alamar blue metabolic assay. Statistical analysis was performed using one-way ANOVA with Tukey’s post-hoc test. *denotes p < 0.05. This figure is adapted from Cutiongco et al., 2014.7
Please click here to view a larger version of this figure.
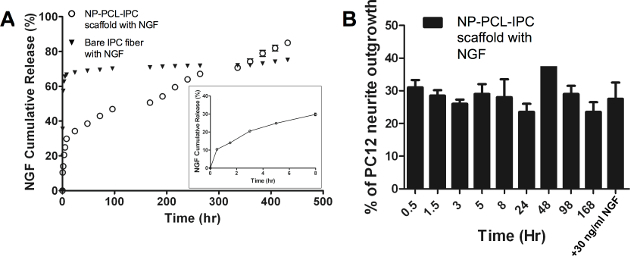 Figure 4. Controlled release and bioactivity of NGF from PCL-IPC composite scaffold. (A) Cumulative release profile of NGF from PCL-IPC composite scaffolds. NGF release was measured at various time points using ELISA specific for NGF. Insert shows cumulative release profile of NGF from PCL-IPC scaffold for the first 8 hr. (B) Bioactivity of NGF as measured by outgrowth of PC12 neural cells. PC12 outgrowth was measured through image analysis. This figure is adapted from Teo et al., 2014.2Please click here to view a larger version of this figure.
Figure 4. Controlled release and bioactivity of NGF from PCL-IPC composite scaffold. (A) Cumulative release profile of NGF from PCL-IPC composite scaffolds. NGF release was measured at various time points using ELISA specific for NGF. Insert shows cumulative release profile of NGF from PCL-IPC scaffold for the first 8 hr. (B) Bioactivity of NGF as measured by outgrowth of PC12 neural cells. PC12 outgrowth was measured through image analysis. This figure is adapted from Teo et al., 2014.2Please click here to view a larger version of this figure.
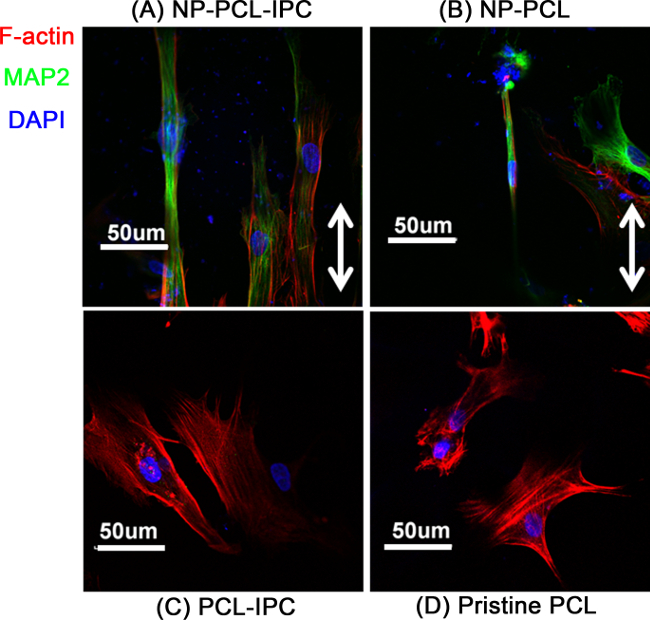 Figure 5. Differentiation of hMSC on NP-PCL-IPC scaffold. Confocal scanning microscopy image of hMSC cultured on different composite scaffolds. (A) NP-PCL-IPC, (B) NP-PCL, (C) PCL-IPC, (D) Pristine PCL scaffolds. Green stain denotes MAP2, a neuronal lineage marker. Red stain denotes F-actin, showing the cellular cytoskeleton. Blue stain denotes the nucleus. This figure is adapted from Teo et al., 2014.2
Please click here to view a larger version of this figure.
Figure 5. Differentiation of hMSC on NP-PCL-IPC scaffold. Confocal scanning microscopy image of hMSC cultured on different composite scaffolds. (A) NP-PCL-IPC, (B) NP-PCL, (C) PCL-IPC, (D) Pristine PCL scaffolds. Green stain denotes MAP2, a neuronal lineage marker. Red stain denotes F-actin, showing the cellular cytoskeleton. Blue stain denotes the nucleus. This figure is adapted from Teo et al., 2014.2
Please click here to view a larger version of this figure.
Table 1. Characteristics of biochemicals used for controlled release from composite IPC scaffolds.
Discussion
IPC fibers are formed by the interaction of two oppositely charged polyelectrolytes. The process utilizes the extraction of the complex from the interface of the polyelectrolytes, facilitating a self-assembly process for stable fiber formation. The mechanism of IPC fiber formation ensures that any biomolecule added into a similarly charged polyelectrolyte can be incorporated during the complexation process.10,11 Conversely, addition of a biomolecule into the oppositely charged polyelectrolyte will result in instantaneous precipitation. The simple fabrication methodology for IPC fibers lends versatility in incorporating various biological materials such as cells, growth factors and small molecules. This critical feature of IPC fibers was also observed in both the PD-IPC and PCL-IPC composite scaffolds, where growth factors with different physical and biomolecular properties (charge and molecular weight) were incorporated with high efficiency. In contrast, encapsulation efficiency using microsphere methodologies for VEGF and NGF can be as low as 16%6 and 8%13, respectively.
Both PD-IPC and PCL-IPC scaffolds also demonstrated temporal control of biomolecule release. VEGF from PD-IPC scaffolds showed linear release for at least 7 days. In contrast, reported VEGF release profiles from polymeric microspheres showed an initial burst release within 24 hr, releasing at least 60% of the total VEGF content.5,14,15 Similarly, NGF was shown to have sustained growth factor delivery from PCL-IPC scaffolds, while other polymeric-based growth factor delivery systems show a plateau of release from 20 days of release.13,16 Presumably, the different components of the composite scaffolds both contribute to growth factor release kinetics. Polymer relaxation mechanisms, porosity and tortuosity can greatly affect the release of hydrophilic biomolecules.17 In addition, the chemical characteristics of the polymeric scaffold may have electrostatic attraction and repulsion towards the growth factors that affects biomolecule release. Thus, the characteristics of the polymeric scaffold are critical in determining release profiles and biomolecules with temporally-controlled release. For instance, while the PD scaffold showed near-linear BSA release, a similar composite scaffold using poly(vinyl alcohol) hydrogel lacked permeability to BSA.18 Polymeric scaffolds that can be used in combination with IPC fibers may require preliminary testing to determine its permeability range.
In addition to controlled biochemical release, the capacity to retain the bioactivity of growth factors is an important feature for any biomolecule delivery system. The harsh alkaline environment of the PD solution and the presence of organic DCM solvent in PCL have the potential to degrade any sort of biomolecules. For example, microsphere delivery system that used dichloromethane showed a consistent trend of diminishing bioactivity with increasing timepoint due to the degradation of NGF.16 Nonetheless, we observed that the bioactivity of VEGF and NGF is maintained, further reiterating that a key advantage of IPC fibers are assimilated within the composite scaffolds.
The use of a sacrificial, biocompatible polysaccharide or polymeric frame that can be easily incorporated into the bulk scaffold gives the versatility to create a composite scaffold with multiple IPC fibers aligned in different configurations.7 Thus, it is necessary that the polymeric scaffolds to be applied with IPC fibers have the capacity for further assimilation into a bulk scaffold. The assimilation process is important in fully embedding IPC fibers into the polymeric scaffold. We observed this phenomenon in the sacrificial pullulan and PCL frames, which were both easily dissolved in the solvent of the bulk PD or PCL scaffold. Furthermore, the single-step method for IPC fabrication and biomolecule incorporation gives flexibility to the number of biomolecules that can be delivered by a single composite scaffold. The simplicity of the methodology also allows fine-tuning of the incorporation efficiency and release kinetics by changing polyelectrolyte identity or concentration. The natural polyelectrolytes chitosan and alginate were used due to its high charge density and similarity to various ECM carbohydrates found in animal tissues. Yet methylated collagen and terpolymer8,19 or chitin and alginate20 may also be used for IPC fiber formation to varying effects. Multiple IPC fibers release different biochemical cues with different kinetics can also be achieved to create a multi-functional composite scaffold. For example, extracellular matrix proteins such as fibronectin loaded into IPC fibers may provide a platform for spatially controlled cell adhesion.7 Sustained release of antibiotics and growth factors are also desirable for tissue regeneration applications. This may be possible by using PCL-IPC, which can incorporate hydrophobic, small molecule drugs and hydrophilic, protein-based growth factors in different compartments of the composite scaffold.2
Our presented methodology also permitted the fabrication of a scaffold with both topographical and biochemical cues. We observed that the NP-PCL-IPC scaffold had the highest enhancement of hMSC differentiation into the neuronal lineage, implying that mimicking multiple aspects of the biophysical and biochemical cellular niche is beneficial in directing cell behavior. The ease of the presented methodology permits its application to other patternable polymeric systems such as polyacrylamide21 or polyethylene glycol22, provided that the crosslinking process will not significantly affect IPC fiber integrity. For example, PD scaffolds were chosen in this study for its unique crosslinking mechanism that occurs in ambient and aqueous conditions.24 This can potentially provide more physiologically relevant substrates for in vitro and in vivo studies. In addition, embedding IPC fibers in a composite scaffold can overcome the lack of mechanical strength of IPC fibers23 by providing tensile strength. Indeed, PCL25 was chosen for its high mechanical strength.
In summary, a simple method for creating composite scaffolds for biomolecule delivery was described. We demonstrated how IPC fibers can be used for sustained delivery of biomolecules without compromising its bioactivity and the incorporation efficiency. We showed this by creating composite scaffolds with two variations: PD-IPC and PCL-IPC scaffolds. Applicability of IPC fibers is not limited to incorporation in PD- and PCL-based scaffolds, but can be potentially extended to other polymeric systems and to deliver other biomolecules.
Disclosures
The authors have nothing to disclose.
Acknowledgments
This work was supported by the Singapore National Research Foundation administered by one of its Research Centers of Excellence, the Mechanobiology Institute, Singapore. MFAC is supported by the Agency for Science, Technology and Research (Singapore) and National Agency for Research (France) joint program under project number 1122703037. BKKT is supported by the Mechanobiology Institute. We thank Mr. Daniel HC Wong for proof-reading the manuscript and Ms. Dawn JH Neo for assisting in the video production.
References
- Annabi N, Tamayol A, et al. 25th Anniversary Article: Rational design and applications of hydrogels in regenerative medicine. Adv. Mater. 2014;26(1):85–124. doi: 10.1002/adma.201303233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teo BKK, Tan GDS, Yim EKF. The synergistic effect of nanotopography and sustained dual release of hydrophobic and hydrophilic neurotrophic factors on human mesenchymal stem cell neuronal lineage commitment. Tissue Eng. Part A. 2014;20(15-16):2151–2161. doi: 10.1089/ten.tea.2013.0382. [DOI] [PubMed] [Google Scholar]
- Lee K, Silva EA, Mooney DJ. Growth factor delivery-based tissue engineering: general approaches and a review of recent developments. J. R. Soc. Interface. 2011;8(55):153–170. doi: 10.1098/rsif.2010.0223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Q, Chen RR, Shen Y, Mooney DJ, Rajagopalan S, Grossman PM. Sustained vascular endothelial growth factor delivery enhances angiogenesis and perfusion in ischemic hind limb. Pharm. Res. 2005;22(7):1110–1116. doi: 10.1007/s11095-005-5644-2. [DOI] [PubMed] [Google Scholar]
- Rui J, Dadsetan M, et al. Controlled release of vascular endothelial growth factor using poly-lactic-co-glycolic acid microspheres: in vitro characterization and application in polycaprolactone fumarate nerve conduits. Acta Biomater. 2012;8(2):511–518. doi: 10.1016/j.actbio.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King TW, Patrick CW. Development and in vitro characterization of vascular endothelial growth factor (VEGF)-loaded poly(DL-lactic-co-glycolic acid)/poly(ethylene glycol) microspheres using a solid encapsulation/single emulsion/solvent extraction technique. J. Biomed. Mater. Res. 2000;51(3):383–390. doi: 10.1002/1097-4636(20000905)51:3<383::aid-jbm12>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Cutiongco MFA, Tan MH, Ng MYK, Le Visage C, Yim EKF. Composite pullulan-dextran polysaccharide scaffold with interfacial polyelectrolyte complexation fibers: A platform with enhanced cell interaction and spatial distribution. Acta Biomater. 2014;10(10):4410–4418. doi: 10.1016/j.actbio.2014.06.029. [DOI] [PubMed] [Google Scholar]
- Yow SZ, Quek CH, Yim EKF, Lim CT, Leong KW. Collagen-based fibrous scaffold for spatial organization of encapsulated and seeded human mesenchymal stem cells. Biomaterials. 2009;30(6):1133–1142. doi: 10.1016/j.biomaterials.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yim EKF, Wan ACA, Le Visage C, Liao IC, Leong KW. Proliferation and differentiation of human mesenchymal stem cell encapsulated in polyelectrolyte complexation fibrous scaffold. Biomaterials. 2006;27(36):6111–6122. doi: 10.1016/j.biomaterials.2006.07.037. [DOI] [PubMed] [Google Scholar]
- Liao IC, Wan AC, Yim EKF, Leong KW. Controlled release from fibers of polyelectrolyte complexes. J. Control. Release. 2005;104(2):347–358. doi: 10.1016/j.jconrel.2005.02.013. [DOI] [PubMed] [Google Scholar]
- Wan ACA, Liao IC, Yim EKF, Leong KW. Mechanism of fiber formation by interfacial polyelectrolyte complexation. Macromolecules. 2004;37(18):7019–7025. [Google Scholar]
- Yim EKF, Reano R, Pang S, Yee A, Chen C, Leong KW. Nanopattern-induced changes in morphology and motility of smooth muscle cells. Biomaterials. 2005;26(26):5405–5413. doi: 10.1016/j.biomaterials.2005.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H, Xu F, Guo D, Yu H. Preparation and evaluation of NGF-microsphere conduits for regeneration of defective nerves. Neurol. Res. 2012;34(5):491–497. doi: 10.1179/1743132812Y.0000000037. [DOI] [PubMed] [Google Scholar]
- Simón-Yarza T, Formiga FR, Tamayo E, Pelacho B, Prosper F, Blanco-Prieto MJ. PEGylated-PLGA microparticles containing VEGF for long term drug delivery. Int. J. Pharm. 2013;440(1):13–18. doi: 10.1016/j.ijpharm.2012.07.006. [DOI] [PubMed] [Google Scholar]
- Patel ZS, Ueda H, Yamamoto M, Tabata Y, Mikos AG. In vitro and in vivo release of vascular endothelial growth factor from gelatin microparticles and biodegradable composite scaffolds. Pharm. Res. 2008;25(10):2370–2378. doi: 10.1007/s11095-008-9685-1. [DOI] [PubMed] [Google Scholar]
- Sun H, Xu F, Guo D, Liu G. In vitro evaluation of the effects of various additives and polymers on nerve growth factor microspheres. Drug Dev. Int. Pharm. 2014;40(4):452–457. doi: 10.3109/03639045.2013.767829. [DOI] [PubMed] [Google Scholar]
- Lee PI. Kinetics of drug release from hydrogel matrices. J. Control. Release. 1985;2:277–288. [Google Scholar]
- Cutiongco MFA, Choo RKT, Shen NJX, Chua BMX, Sju E, Choo AWL, Le Visage C, Yim EKF. Composite scaffold of poly(vinyl alcohol) and interfacial polyelectrolyte complexation fibers for controlled biomolecule delivery. Front. Bioeng. Biotechnol. 2015;3(3):1–12. doi: 10.3389/fbioe.2015.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yow SZ, Lim TH, Yim EKF, Lim CT, Leong KW. A 3D Electroactive Polypyrrole-Collagen Fibrous Scaffold for Tissue Engineering. Polymers. 2011;3(1):527–544. [Google Scholar]
- Leong MF, Toh JKC, et al. Patterned prevascularised tissue constructs by assembly of polyelectrolyte hydrogel fibres. Nat. Commun. 2013;4:2353. doi: 10.1038/ncomms3353. [DOI] [PubMed] [Google Scholar]
- Di Benedetto F, Biasco A, Pisignano D, Cingolani R. Patterning polyacrylamide hydrogels by soft lithography. Nanotechnology. 2005;16(5):S165. [Google Scholar]
- Revzin A, Russell RJ, et al. Fabrication of poly(ethylene glycol) hydrogel microstructures using photolithography. Langmuir. 2001;17(18):5440–5447. doi: 10.1021/la010075w. [DOI] [PubMed] [Google Scholar]
- Yim EKF, Liao IC. Tissue compatibility of interfacial polyelectrolyte complexation fibrous scaffold: evaluation of blood compatibility and biocompatibility. Tissue Eng. Part A. 2007;13(2):423–433. doi: 10.1089/ten.2006.0113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaouat M, Le Visage C, Autissier A, Chaubet F, Letourneur D. The evaluation of a small-diameter polysaccharide-based arterial graft in rats. Biomaterials. 2006;27:5546–5553. doi: 10.1016/j.biomaterials.2006.06.032. [DOI] [PubMed] [Google Scholar]
- Eshraghi S, Das S. Mechanical and microstructural properties of polycaprolactone scaffolds with one-dimensional, two dimensional, and three-dimensional orthogonally oriented porous architectures produced by selective laser sintering. Acta Biomater. 2010;6:2467–2476. doi: 10.1016/j.actbio.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]


