Abstract
In recent years, increasing attention has been paid to pulmonary hypertension (PH) as a strong and independent risk factor for adverse outcome in the population of patients on long-term dialysis.
Published results of observational studies indicate that the problem of PH refers mostly to patients on long-term hemodialysis and is less common in peritoneal dialysis patients. The main cause of this complication is proximal location of the arteriovenous fistula, causing chronically increased cardiac output.
This paper presents the usefulness of transthoracic echocardiography (TTE) for measurement of the Tricuspid Annular Plane Systolic Excursion (TAPSE) in the early diagnosis of PH in dialysis patients.
Echocardiographic diagnosis of pulmonary hypertension with TTE, especially in the case of HD patients, ensures the selection of the proper location for the first arteriovenous fistula and facilitates the decision to switch to peritoneal dialysis or to accelerate the process of qualification for kidney transplantation.
MeSH Keywords: Echocardiography, Doppler; Hypertension, Pulmonary; Kidney Failure, Chronic; Peritoneal Dialysis
Background
Thanks to technological progress, in the last 30 years there has been a significant increase in the number of patients with ESRD (end-stage renal disease) undergoing renal replacement therapy. Despite this progress, recent statistics show that technological advancement does not translate into reduced mortality or morbidity in chronic dialysis patients. Yearly mortality in the group of patients with chronic kidney failure in stage 5 D varies from 6.6% in Japan, 15.6% in Europe, and 21.7% in the USA. Although most therapy-optimizing guidelines were implemented with maximum efficiency, morbidity of hemodialysis patients in the USA decreased by only 1% per decade and the average duration of hospitalization did not fall below 15 days per year [1].
This data indicates the need for early identification of classic risk factors for cardiovascular diseases such as coronary heart disease and chronic heart failure with arrhythmic complications, as recognized predictors of fatal prognosis in dialysis patients with ESRD [2].
In recent years, substantial attention was paid to the development of pulmonary hypertension (PH), as another strong risk factor for adverse outcomes in a population of patients on both hemodialysis (HD) and peritoneal dialysis program (PD) [3]. It has been observed by DiLullo et al., who defined the PH with an associated right ventricle failure as a “new challenge for cardionephrologists in the 21st century” [4].
Our article presents the usefulness of echocardiography in the early diagnosis of PH in dialysis patients, based on our own clinical experience.
Pulmonary Hypertension, and Chronic Kidney Disease: Whether and What’s New?
Common guidelines of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS) for the diagnosis and treatment of pulmonary hypertension define it as the pathological and hemodynamic state characterized by elevated mean pulmonary artery pressure ≥25 mmHg at rest diagnosed with right heart catheterization (RHC). Pulmonary hypertension occurs in the course of a number of diseases qualified by ESC and ERS to five categories covering 37 clinical units of different pathogenesis and clinical course, as well as therapy [5].
Thus, group 1 includes pulmonary arterial hypertension (mainly idiopathic and genetically determined); group 2 – PH due to left heart diseases; group 3 – PH due to lung diseases; group 4 – chronic thromboembolic pulmonary hypertension; group 5 – pulmonary hypertension of unclear and/or multifactorial pathogenesis, including chronic kidney disease (CKD).
Havlucu et al. in their prospective study in which 211 patients with ESRD were enrolled (56% undergoing hemodialysis), diagnosed 48 cases of PH [6]. Similar results were achieved by Abdelwhab and Elshinawy, who in the group of 76 patients with ESRD recognized pulmonary hypertension in 32% of patients treated conservatively and in 44% of hemodialyzed patients [7]. Paneni et al., analyzing the incidence of echocardiographic signs of right ventricular dysfunction in a population of 120 dialyzed patients demonstrated that those abnormalities occur significantly more often in patients treated with hemodialysis (HD) than peritoneal dialysis (71% vs. 35%) [8]. Kawar et al. analyzed the results of 13 observational studies on echocardiographic diagnosis of pulmonary hypertension in ESRD patients and concluded that this problem occurred in 30–60% patients treated with HD. However, in the population of patients on PD, pulmonary hypertension was diagnosed less frequently – in 12 to 42% of patients, which was comparable with the results obtained in a population of CKD patients treated conservatively. In the latter group, PH was diagnosed in 13 to 39% of patients [9]. Regarding patients undergoing HD, creation of proximal arteriovenous fistula and chronically increased cardiac output (AVF) are considered the main cause of PH [8]. Proximal location of AVF compared to its distal location might cause blood flow elevated up to 1.0–1.5 l/min through extracorporeal circulation and consequently, recirculation of blood through the pulmonary vascular bed increase up to 20%, which additionally causes right heart volume overload in hemodialysis patients [10,11]. According to Amerling et al. hemodynamic consequences of AVF responsible for the development of PH are increased blood flow and pressure in the pulmonary circulation, as the consequences of an increase in cardiac output, decrease of peripheral resistance and increased activity of the sympathetic nervous system [12].
Potential risk factors are presented in Table 1 [13].
Table 1.
Potential risk factors for the development of pulmonary hypertension in end-stage renal diseases patients (ESRD) according to Sofia and Stanziola [12].
| Risk factors for PH in ESRD patients | Effect |
|---|---|
| Arteriovenous fistula | ↑ CO |
| Endothelial dysfunction | ↓ NO, ↑ TXB, ↑ ET-1 |
| Dysfunction of the left ventricle | ↑ PWP |
| Endocrine abnormalities | Hyperparathyroidism |
| Gas exchange abnormalities | Hypoxia, pulmonary vascular vasoconstriction |
CO – cardiac output; NO – nitric oxide; TXB – thromboxane; ET-1 – endothelin-1; PWP – pulmonary wedge pressure.
The Role of Echocardiography in the Diagnosis of Pulmonary Hypertension
The right heart catheterization (RHC) remains a “gold standard” for the diagnosis of PH, but as an invasive test is available only in specialized clinical centers. In accordance with the guidelines of the European Society of Cardiology from 2009, PH can be identified when the test shows the average resting mean pulmonary artery pressure ≥25 mmHg (mPAP) [5]. Taking into account the non-characteristic clinical manifestation of PH in the form of exertional dyspnea and weakness, before qualifying the patient for RHC, the initial diagnosis of PH should be confirmed by non-invasive diagnostic tests. This restricts the population to the group of patients suspected, in which invasive diagnostic is fully justified. In everyday clinical practice, transthoracic echocardiography (TTE) is the most frequently used for this purpose non-invasive diagnostic test. Eugene Braunwald, in a lecture delivered on 09/02/2013 at the Congress of the European Society of Cardiology in Amsterdam counted echocardiography among ten greatest achievements of modern cardiology [14]. Contemporary echocardiography is becoming available not only in specialized institutes, but also directly at patients’ bedside. This diagnostic method allows non-invasive assessment of pressure in the pulmonary circulation and imaging of morphological, functional as well as hemodynamic consequences of PH. Finally, it is possible to analyze simultaneously the severity of PH and differentiate its potential causes.
According to the cited ESC recommendations, the echocardiography should be prescribed for all patients with suspected PH [5]. Available modes of echocardiographic imaging, ranging from one-dimensional echocardiography, through two-dimensional, three-dimensional, as well as the Doppler techniques, intravascular and intracardiac echocardiography, answer most questions about the structure and function of myocardium, as well as major vessels of the chest. What is important from a practical point of view, the patient does not need to be specially prepared for the TTE, which significantly simplifies the process of diagnosis and treatment. It should be noted that computed tomography often preferred to echocardiography is associated with both short-term risk of complications (allergic reactions to the contrast agent, the deterioration of renal function) and long term reactions related to ionizing radiation [15].
In summary, modern echocardiography allows simultaneous estimation of many indicators of right ventricular function and pulmonary circulation and should be carried out as a first-line test in every patient with suspicion of pulmonary hypertension [5].
Echocardiographic Basics Assessment of Pulmonary Hypertension
Doppler assessment of pulmonary artery systolic pressure (PASP) which is synonymous – after exclusion of pulmonic stenosis – with right ventricular systolic pressure (RVSP) remains the basis of echocardiographic evaluation of pulmonary pressure and right ventricular function.
Using the tricuspid regurgitant jet velocity recorded with continuous wave Doppler, the Bernoulli equation (maximum gradient=4V2) is used to obtain the right ventricular to right atrial systolic pressure gradient (Tricuspid Regurgitation Peak Gradient, TRPG). TRPG value >31 mmHg (i.e. tricuspid regurgitation velocity >2.8 m/s) suggests PH.
In turn, the value of RVSP (allowing the estimation of PASP) is obtained by adding to the TRPG value of RAP (pressure in the right atrium of the heart):
The RAP estimation is on the diameter and respiratory variability of the inferior vena cava (IVC). Thus, inferior vena cava collapsing about 50% during inspiration with a width less than 15 mm indicates the proper RAP (about 5 mm Hg). Inferior vena cava with the width reduction ≥15mm or without inspiratory collapse calls for RAP increase by more than 20 mm Hg. If calculated according to the above presented principle RVSP value exceeds 35–40 mmHg then patient requires a more detailed assessment towards PH.
Due to the relative simplicity of this method in clinical practice the tricuspid regurgitant jet in conjunction with an estimate of right atrial pressure is used in most laboratories to calculate pulmonary artery systolic pressure [5,16].
Figure 1 shows principle of RVSP assessment using continuous Doppler wave apical four-chamber projection.
Figure 1.
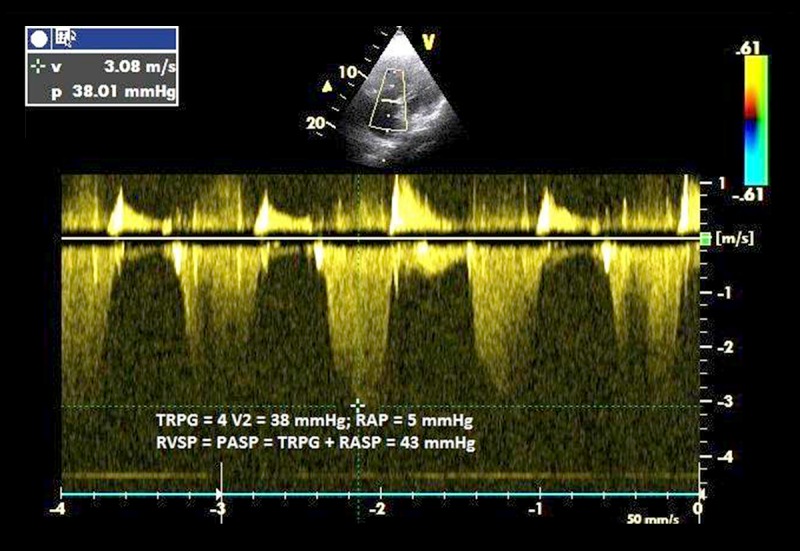
In this 64-year-old man with chronic obstructive pulmonary disease, the tricuspid regurgitant jet velocity of 3.08 m/s predicts a right ventricular to right atrial pressure gradient (TRPG) of 38 mmHg. In combination with assessment of right atrial pressure (RAP), based on the respiratory variation in the size of the inferior vena cava, the estimated pulmonary systolic pressure (PASP) in this patient is 43 mmHg.
It is worth noting that the only hemodynamic parameter included in the definition of PH is the mean value of pulmonary artery pressure (mPAP). Its estimation using PASP value assessed with Doppler echocardiography is obtained through a number of formulae. One of them developed by a group of French researchers (Chemla D, Castelain V, Humbert M. et al.) is as follows [17]:
Other Doppler methods for estimating pulmonary artery pressure include measurements of early- and late-diastolic regurgitant velocity on the level of the pulmonary trunk valve and the time to peak velocity in the pulmonary outflow tract (also called acceleration time – AcT). In the case of the latter parameter shortening of the AcT<60 msec at moderately elevated PASP (<60 mmHg) is one of the specific echocardiographic indicators of acute pulmonary embolism [18].
The limitations of Doppler technique measurement of the pressure in the pulmonary artery are considerable. This test does not directly measure the pressure in the pulmonary arteries and may be inaccurate, particularly in the case of impaired function of the right heart or cardiac arrhythmias. In the first case, the systolic right ventricular dysfunction and elevated right atrial pressure will result in lower systolic gradient between these cavities and underestimation of pulmonary hypertension values [5,16]. In contrast, atrial fibrillation with a high ventricular rate or other cardiac arrhythmias can cause large fluctuations in the TRPG values, which depend on the variable time of the right ventricle filling. The use of the aforementioned method in patients with arrhythmias is associated with frequent overestimation of PASP, occurring in up to 50% of investigated patients [19].
Therefore, according to the ESC guidelines for the diagnosis and treatment of pulmonary hypertension echocardiography can only determine the probability of the occurrence of PH, and not its actual severity [5].
Table 2 shows the parameters defining the low, moderate, and high probability of PH based on echocardiography.
Table 2.
Assessment of the likelihood of pulmonary hypertension (PH) based on echocardiography according to ESC and ERS recommendations [5].
| Parameter | low | Moderate | High | |
|---|---|---|---|---|
| TRV | ≤2.8 m/s | ≤2.8 m/s | 2.9–3.4 m/s | >3.4 m/s |
| TRPG | ≤31.4 mmHg | ≤31.4 mmHg | 33.6–46 mmHg | >46 mmHg |
| PASP=RVSP | ≤36 mmHg | ≤36 mmHg | 36–50 mmHg | >50 mmhg |
| other echocardiographic markers of | ||||
| PH: evaluation of blood flow through the pulmonary valve; evaluation of overload and function of the right ventricle |
absent | present | present or absent | present or absent |
TRV – maximum velocity of tricuspid regurgitation; TRPG – the tricuspid regurtitation pique gradient; PASP – systolic pulmonary artery pressure; RVSP – systolic pressure in the right ventricle.
In every patient with clinically suspected PH, a necessary element of echocardiographic examination is to assess the function of right ventricle and degree of its overload. Suspicion of the PH suggests the presence of increased right ventricular dimensions with flattening, displacement, and paradoxical movement of the interventricular septum (IVS) in the direction of the left ventricle.
Thickening of the right ventricle walls and the pulmonary artery trunk extension also suggest PH, but appear in the later stages of this pathology [16]. It should be emphasized that the role of echocardiographic assessment of morphological changes in the diagnosis of PH increases in cases of unreliable pressure measurement with Doppler method (e.g. in arrhythmias with high ventricular rate).
According to the current guidelines for the assessment of right ventricular systolic function the following echocardiographic parameters should be used:
the amplitude of tricuspid annular plane systolic excursion (TAPSE);
the percentage of fractional area change (FAC);
the measurement of the longitudinal velocity of excursion of the tricuspid annulus and the basal free wall segment of the right ventricle (RV S’) with tissue Doppler echocardiography (TDE).
Tricuspid annular plane systolic excursion (TAPSE), described by Kaul in 1984 and introduced to the clinical practice by Hammarstrom in 1991, is a measurement of M-mode echocardiography that reflects longitudinal contraction of the right ventricle and is the easiest, well documented, quantitative index of right ventricular function. TAPSE values <16–20 mm are considered abnormal [20,21].
Samad et al. in their study of 194 patients with acute myocardial infarction found that the value of TAPSE <15 mm is associated with a significantly higher risk of death (45%) in the two year follow-up compared to patients with TAPSE >20 mm (4%) [22]. TAPSE and FAC measurements significantly correlated with the value of the ejection fraction of the right ventricle assessed with magnetic resonance and radioisotope examinations.[23]
The FAC values <31–35% indicate dysfunction of the right ventricle. It should be noted, however, that the time-consuming measurement of FAC makes it of limited clinical utility.
Tissue Doppler echocardiography (TDE), which recently becomes more available, may also be used to assess the systolic function of the right ventricle. The reduced <10–12 cm/s maximum speed of tricuspid annular motion (S’) is a symptom of right ventricle dysfunction [5]. It should be noted that due to the complicated geometry of right ventricle cavity, the measurement of right ventricular ejection fraction, in contrast to the left ventricle, cannot be applied in everyday practice.
TAPSE as a new diagnostic and prognostic marker of pulmonary hypertension in dialyzed patients
Measurement of TAPSE represents a novelty in the diagnosis and monitoring of PH in dialyzed patients. TAPSE is simple, less dependent on optimal image quality, and reproducible. Implementation of this technique into everyday practice does not require sophisticated equipment or prolonged image analysis and is fairly simple even for less experienced physicians.
Another advantage is the fact that TAPSE can be determined in patients with atrial fibrillation with a high ventricular rate in whom reliable echocardiographic examination, including Doppler measurements, may be difficult to perform.
Figure 2 shows the principle of measuring TAPSE.
Figure 2.
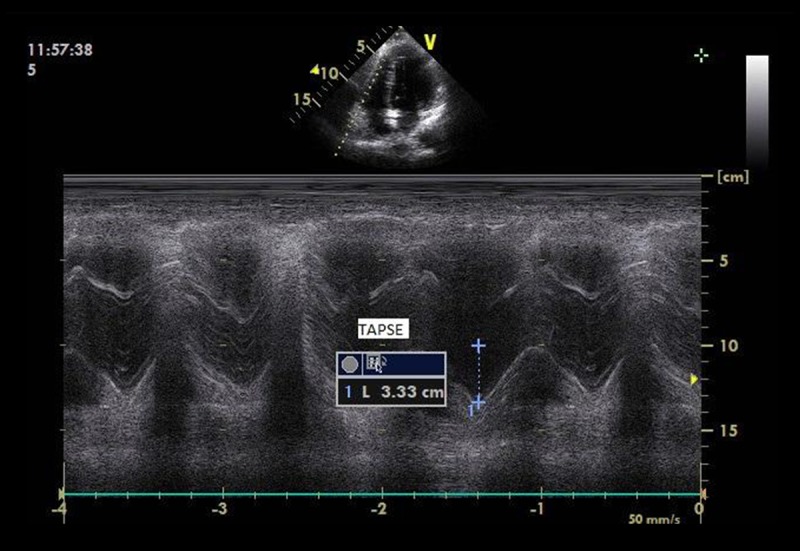
Measurement of Tricuspid Annular Plane Systolic Excursion (TAPSE); acquisitioning M-mode from standard apical four-chamber window; RA – right atrium; RV – right ventricle; lines indicate the course of the ultrasound beam through the lateral part of the tricuspid annulus based on a 2D image.
TAPSE should be measured in the apical four-chamber projection using a one-dimensional echocardiography (M-mode). TAPSE value determines the range of motion or displacement of the tricuspid valve ring in the longitudinal plane in successive phases of the cardiac cycle, reflecting the function of the right ventricle. The normal value of TAPSE is 24±4 mm. Values less than 16 mm indicate impaired systolic function of the right ventricle [5,16]. Forfia et al. in a prospective study of 63 patients diagnosed with PH showed that the value of TAPSE less than 18 mm was an independent risk factor for mortality in this population [24].
Table 3 summarizes the normal values of basic echocardiographic measurements for assessing the structure and function of the right heart.
Table 3.
Normal values for the basic echocardiographic measurements for assessing structures and functions of right heart according to ESD and ERS recommendations [5,16].
| Parameter | Norm |
|---|---|
| RV width in LAX | 0.9–2.6 cm |
| RV width below the PV ring in SAX | 2.5–2.9 cm |
| RV width below the TV ring in 4C | 2.7–3.3 cm |
| RV wall thickness | <0.5 cm |
| Width of the pulmonary trunk | 1.5–2.1 cm |
| IVC width | < 1.7 cm |
| IVC respiratory susceptibility | >50% (during inspiration) |
| TRV (TRPG) | ≤2.8 m/s (31 mm Hg) |
| PV flow rate | 0.6–0.9 m/s |
| AcT spectrum of the flow through the PV | >80 msec |
| TAPSE | 1.5–2.0 cm |
RV – right ventricle; LAX – projection parasternal long axis view; PV – pulmonary valve; SAX – projection parasternal short axis; TV – tricuspid valve; 4C – apical four-chamber projection; IVC – the lower vena cava; TRV – tricuspid regurgitant velocity; TRPG – the maximum gradient of tricuspid regurgitation; AcT – acceleration time; TAPSE – the amplitude of movement of the tricuspid valve ring.
TAPSE in the diagnosis and monitoring of pulmonary hypertension in CKD and hemodialyzed patients
Floccari et al., in a group of 202 patients with stage 3 CKD and a negative history of PH discovered reduced values of TAPSE (<23 mm) in 43% of the studied group. TAPSE showed a statistically significant negative correlation with the values of systolic blood pressure in the pulmonary artery. Consequently, statistical analysis enabled isolation of subpopulation of patients at risk of developing pulmonary hypertension. This group was characterized by low values of TAPSE (around 16 mm) and high values of systolic blood pressure in the pulmonary artery (about 38 mmHg). It should be pointed out, that the study by Floccari et al. included patients in the early stages of CKD, which means not on dialysis. The strength of this study was the identification a subpopulation of patients at risk of developing PH. According to the authors these patients in the case of sudden progression of CKD and need for dialysis should not be qualified to proximal, i.e., high AVF but either for a distal AVF, or for peritoneal dialysis. The authors noted also that in the case of congestive heart failure, shortness of breath and a decrease in daily diuresis, aside from an effect of a decrease in glomerular filtration rate (GFR), may suggest PH [25].
Di Lullo et al. presented in 2011 the results of TAPSE in hemodialysis patients. The study included 20 hemodialysis patients aged 51±10 years with mean time on dialysis 24±8 months, without prior medical history of cardiovascular diseases. The authors observed a significantly lower value of TAPSE (<15 mm) and larger dimensions of the right heart chambers in patients with proximal AVF compared with a group of 10 patients with hemodialysis access consisting of permanent catheter inserted through the internal jugular vein to the superior vena cava. According to Doppler echocardiography in the population of patients with proximal AVF single session of hemodialysis did not affect the pressure in the pulmonary artery, but was associated with a decrease in the value of TAPSE (16±3 mm). Authors interpreted it as an expression of increased preload in the pulmonary circulation. The results obtained by Di Lullo et al. suggest a significant correlation between the type of AVF and pathogenesis of PH in hemodialysis patients [26].
Similar conclusions emerge from the study by Beigi et al. In the group of 34 hemodialysis patients high flow AVF formed on the brachial artery (30 patients; mean blood flow1463 ml/min) showed a significantly higher pulmonary artery pressure in the Doppler assessment in relation to the low flow AVF formed on the radial artery (4, patients; mean blood flow 422 ml/min) [27].
Echocardiographic evaluation of pulmonary hypertension in patients on peritoneal dialysis
The available literature does not provide much data on the prevalence of PH in a population of patients with ESRD treated with peritoneal dialysis. Bozabas et al., in 2009, analyzed a population of 500 dialyzed patients including 432 on hemodialysis and 68 on peritoneal dialysis. Echocardiographic diagnosis of PH was made in 17% (n=85) of all examined patients. The average value of PASP was 46.7 ±8.7 mmHg. Pulmonary hypertension was more frequently diagnosed among hemodialysis patients (19%, n=81) than in patients treated with peritoneal dialysis (6%, n=4) [28]. Similar conclusions were made by Etemadi et al. Their 2012 study included 66 dialyzed patients (mean age 57 years, 34 patients treated with hemodialysis, 32 with peritoneal dialysis) [29]. The mean duration of treatment on dialysis for both groups was respectively 102 and 44 weeks. Echocardiographic features of PH defined as PASP ≥35 mmHg (mean PASP was 37.5 mmHg) were found in 30% of a group (n=20). PH occurred significantly more often in patients on hemodialysis (41%, n=14) compared to patients treated with peritoneal dialysis (19%, n=6) (p=0.04). Among hemodialysis patients diagnosed with PH dominated proximal type of AVF (57%).
Conclusions
The achievements of contemporary echocardiography, especially the introduction of measurement of TAPSE in the early diagnosis of pulmonary hypertension in a population of patients with chronic kidney disease, particularly ESRD requiring dialysis, showed a significant diagnostic and prognostic value of this simple test. In patients with ESRD at risk of developing pulmonary hypertension, echocardiography allows the selection of the proper location for the first arteriovenous fistula. If PH was diagnosed in a patient treated with HD, it facilitates a decision to change the treatment modality to peritoneal dialysis or qualification for earlier kidney transplantation.
Footnotes
Source of support: Departmental sources
References
- 1.Wańkowicz Z. The role of technological progress vs. accidental discoveries and clinical experience in the evolution of dialysis. Med SciMonit. 2013;19:984–92. doi: 10.12659/MSM.889710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sarnak MJ, Levey AS, Schoolwerth AC, et al. Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Reasearch, Clinical Cardiology, and Epidemiology and Prevention. Circulation. 2003;108:2154–69. doi: 10.1161/01.CIR.0000095676.90936.80. [DOI] [PubMed] [Google Scholar]
- 3.Yigla M, Fruchter O, Aharonson D, et al. Pulmonary hypertension is an independent predictor of mortality in hemodialysis patients. Kidney Int. 2009;75:969–75. doi: 10.1038/ki.2009.10. [DOI] [PubMed] [Google Scholar]
- 4.Di Lullo L, Floccari F, Rivera R, et al. Pulmonary hypertension and right heart failure in chronic kidney disease: new challenge for 21st-century cardionephrologists. Cardiorenal Med. 2013;3:96–103. doi: 10.1159/000350952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Galie’N, Hoeper MM, Humbert M, et al. Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J. 2009;30:2493–537. doi: 10.1093/eurheartj/ehp297. [DOI] [PubMed] [Google Scholar]
- 6.Havlucu Y, Kursat S, Ekmekci C, et al. Pulmonary hypertension in patients with chronic renal failure. Respiration. 2007;74:503–10. doi: 10.1159/000102953. [DOI] [PubMed] [Google Scholar]
- 7.Abdelwhab S, Elshinawy S. Pulmonary hypertension in chronic renal failure patients. Am J Nephrol. 2008;28:990–97. doi: 10.1159/000146076. [DOI] [PubMed] [Google Scholar]
- 8.Paneni F, Gregori M, Ciavarella GM, et al. Right ventricular function in patients with end-stage renal disease. Am J Nephrol. 2010;32:432–38. doi: 10.1159/000320755. [DOI] [PubMed] [Google Scholar]
- 9.Kawar B, Ellarn T, Jackson C, Kiley DG. Pulmonary hypertension in renal disease: epidemiology, potential mechanisms and implications. Am J Nephrol. 2013;37:281–90. doi: 10.1159/000348804. [DOI] [PubMed] [Google Scholar]
- 10.Beigi AA, Sadeghi AM, Khosravi AR, et al. Effects of the arteriovenous fistula on pulmonary artery pressure and cardiac output in patients with chronic renal failure. J Vasc Access. 2009;10:160–66. doi: 10.1177/112972980901000305. [DOI] [PubMed] [Google Scholar]
- 11.Unal A, Tasdemir K, Oymak S, et al. The long-term effects of arteriovenous fistula creation on the development of pulmonary hypertension in hemodialysis patients. Hemodial Int. 2010;14:398–402. doi: 10.1111/j.1542-4758.2010.00478.x. [DOI] [PubMed] [Google Scholar]
- 12.Amerling R, Ronco C, Kuhlmann M, et al. Arteriovenous fistula toxicity. Blood Purif. 2001;31:113–20. doi: 10.1159/000322695. [DOI] [PubMed] [Google Scholar]
- 13.Sofia M, Stanziola AA. Kidney pulmonary hypertension: another road on the map? Multidiscip Respir Med. 2011;3:150–52. doi: 10.1186/2049-6958-6-3-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van der Wall EE. Milestones in cardiovascular medicine: 10 or more? Neth Heart J. 2013;21:527–29. doi: 10.1007/s12471-013-0480-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lipiec P, Płońska-Gościniak E, Kuśmierek J, et al. Safety of non-invasive cardiovascular imaging techniques. Expert consensus statement of the Polish Clinical Forum for Cardiovascular Imaging. Kardiologia Polska. 2013;3:301–7. doi: 10.5603/KP.2013.0048. [DOI] [PubMed] [Google Scholar]
- 16.Rudski LG, Lai WW, Afilalo J, et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2010;23:685–713. doi: 10.1016/j.echo.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 17.Chemla D, Castelain V, Humbert M, et al. New formula for predicting mean pulmonary artery pressure using systolic pulmonary artery pressure. Chest. 2004;126:1313–17. doi: 10.1378/chest.126.4.1313. [DOI] [PubMed] [Google Scholar]
- 18.Kurzyna M, Torbicki A, Pruszczyk P, et al. Disturbed right ventricular ejection pattern as a new Doppler echocardiographic sign of acute pulmonary embolism. Am J Cardiol. 2002;90:507–11. doi: 10.1016/s0002-9149(02)02523-7. [DOI] [PubMed] [Google Scholar]
- 19.Fisher MR, Forfia PR, Chamera E, et al. Accuracy of Doppler echocardiography in the hemodynamic assessment of pulmonary hypertension. Am J ResprCrit Care Med. 2009;179:615–21. doi: 10.1164/rccm.200811-1691OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaul S, Tei C, Hopkins JM, et al. Assessment of right ventricular function using two-dimensional echocardiography. Am Heart J. 1984;107:526–31. doi: 10.1016/0002-8703(84)90095-4. [DOI] [PubMed] [Google Scholar]
- 21.Hammarstrom E, Wranne B, Pinto FJ, et al. Tricuspid annular motion. J Am Socechocardiogr. 1991;4:131–39. doi: 10.1016/s0894-7317(14)80524-5. [DOI] [PubMed] [Google Scholar]
- 22.Samad BA, Alam M, Jensen-Urstad K. Prognostic impact of right ventricular involvement as assessed by tricuspid annular motion in patients with acute myocardial infarction. Am J Cardiol. 2002;90:778–81. doi: 10.1016/s0002-9149(02)02612-7. [DOI] [PubMed] [Google Scholar]
- 23.Anavekar NS, Gerson D, Skali H, et al. Two-dimensional assessment of right ventricular function: an echocardiographic-MRI correlative study. Echocardiography. 2007;24:452–56. doi: 10.1111/j.1540-8175.2007.00424.x. [DOI] [PubMed] [Google Scholar]
- 24.Forfia PR, Fisher MR, Mathai SC, et al. Tricuspid annular displacement predicts survival in pulmonary hypertension. Am J RespirCrit Care Med. 2006;174:1034–41. doi: 10.1164/rccm.200604-547OC. [DOI] [PubMed] [Google Scholar]
- 25.Floccari F, Granata A, Rivera R, et al. Echocardiography and right ventricular function in NKF stage III chronic kidney disease: Ultrasound nephrologists’role. J Ultrasound. 2012;15:252–56. doi: 10.1016/j.jus.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Di Lullo L, Floccari F, Polito P, et al. Right ventricular diastolic function in dialysis patients could be affected by vascular access. Nephron Clin Pract. 2011;118:c258–62. doi: 10.1159/000321867. [DOI] [PubMed] [Google Scholar]
- 27.Beigi AA, Sadeghi AM, Khosravi AR, et al. Effects of the arteriovenous fistula on pulmonary artery pressure and cardiac output in patients with chronic renal failure. J Vasc Access. 2009;10:160–66. doi: 10.1177/112972980901000305. [DOI] [PubMed] [Google Scholar]
- 28.Bozabas SS, Akcay S, Altin C, et al. Pulmonary hypertension in patients with end-stage renal disease undergoing renal transplantation. Transplant Proc. 2009;41:2753–56. doi: 10.1016/j.transproceed.2009.07.049. [DOI] [PubMed] [Google Scholar]
- 29.Etemadi J, Zolfaghari H, Firoozi R, et al. Unexplained pulmonary hypertension in peritoneal dialysis and hemodialysis patients. Rev Port Pneumol. 2012;18(1):10–14. doi: 10.1016/j.rppneu.2011.07.002. [DOI] [PubMed] [Google Scholar]


