Abstract
The prognosis of metastatic non-small cell lung cancer (NSCLC) is poor, and platinum-based chemotherapy improves the median survival for only a few months. A subgroup of patients with oligometastatic disease may benefit from surgical resection, but only very limited data are available to date. We conducted a retrospective review of all patients with synchronous extrapulmonary oligometastatic NSCLC undergoing surgical resection in our department. Data regarding medical history, histology, number of metastases, and survival status were extracted from the medical database of the University Medical Center, Freiburg. Fifty-six patients underwent surgical resection for oligometastatic lung cancer. Five patients were lost during follow-up and therefore censored. One patient died perioperatively due to acute respiratory distress syndrome. The remaining 50 patients had an overall median survival time of 14.6 months. Analyzing the influence of metastatic site, we found a median overall survival of 23.4 months for patients with soft tissue metastasis, 16.7 months for patients with brain metastasis, 9.5 months for patients with adrenal gland involvement, and only 4.3 months for patients with bone metastasis (p < 0.005). Upon multivariate analysis, bone metastasis was the only significant parameter influencing median overall survival (p < 0.004). Based on our data, we conclude that an aggressive surgical approach for oligometastatic NSCLC can be performed with acceptable mortality and morbidity. In this rare constellation, surgical therapy may be an option in selected cases.
Keywords: NSCLC, Single metastasis, Stage, Skip metastasis
Introduction
Lung cancer is one of the most common causes of cancer-related death and is a malignancy with high incidence worldwide [1]. Approximately 80 % of all lung cancers are diagnosed histologically as non-small cell lung cancer (NSCLC), and at the time of diagnosis, a high proportion of patients already suffer from metastatic disease. The median overall survival of patients with Union Internationale Contre le Cancer (UICC) stage IV cancer is only 6 months [2]. Standard treatment of metastatic lung cancer involves systemic chemotherapy, and a platinum-based two-drug combination is recommended as first-line therapy in most cases [3]. Unfortunately, this improves overall survival for only a few months [2, 4]. However, 7 % of patients with metastatic disease only suffer from a single metastasis; in 1995, Hellman and Weichselbaum introduced the term “oligometastatic” for this special subgroup of patients [5, 6]. These patients may benefit from surgical resection of the primary tumor and its metastasis. Several retrospective case studies have documented an improved outcome in patients treated surgically, but most studies reported only on isolated metastasis of the brain or adrenal gland [7–10]. Therefore, it is unknown whether patients with an isolated metastasis in other locations also benefit from this aggressive approach. Recently, Salah et al. reviewed 51 cases to analyze the outcome of extrapulmonary, extracerebral, and extra-adrenal oligometastatic NSCLC [7–11]. The 5-year overall survival rate was 50 %, which implies a high publication bias. To investigate the clinical outcomes of oligometastatic NSCLC and the factors influencing median survival, we analyzed the complete spectrum of patients with oligometastatic NSCLC treated in our department.
Materials and Methods
Definition of “Oligometastatic”
The definition of the term “oligometastatic” is not precise in literature and ranges between the description of one and five metastases. This is at least arbitrary, and it is not clear where to draw the line between oligometastatic and metastatic lung cancer. Weichselbaum et al. introduced this term to indicate that there is an intermediate state of lung cancer between metastatic and local disease. Therefore, we decided to follow the inclusion criteria of Hasselle et al. and included all patients with ≤5 extrapulmonary metastases [12].
Patients and Surgical Techniques
We conducted a retrospective review of 56 patients with synchronous extrapulmonary oligometastatic NSCLC undergoing surgical resection in our department between 1987 and 2011. Data regarding medical history, histology, number of metastases, and survival status were extracted from the medical database of the University Medical Center, Freiburg. Patient stage was reviewed according to the sixth TNM classification of the UICC [13]. Histopathological findings were classified according to World Health Organization criteria [14]. All patients underwent a complete staging procedure preoperatively, including at least computed tomography (CT) or magnetic resonance imaging of the brain, CT of the chest and upper abdomen, bronchoscopy, ultrasonography of the abdomen, bone scintigraphy, and/or F-fluorodeoxyglucose positron emission tomography CT. In all cases, the staging procedure also included cytological or histological investigation of mediastinal lymph nodes. Lung function analysis, including spirometry and blood gases, was also routinely performed. Thoracotomies were performed with a standardized anterolateral approach.
Follow-up
Overall survival was calculated beginning with the date of diagnosis. Whenever possible, basic information was retrieved from medical records of the University Medical Center, Freiburg, from general practitioners, or by telephone interviews. Cross-sectional contact for all surviving patients was performed in October 2011. Mean follow-up time was 24.3 months.
Statistical Analysis
Statistical analysis was conducted by using MedCalc software Version 11.6.1.0 (MedCalc software, Mariakerke, Belgium). Survival curves were constructed according to the Kaplan–Meier method. Differences in survival were analyzed by log-rank test. Multivariate analysis was performed by using the Cox proportional hazards model.
Ethics Statement
This was designed as a retrospective study, and all patients (treated in the Comprehensive Cancer Center, Freiburg) provided written informed consent that their medical data could be used for scientific reasons, while maintaining patient confidentiality. This study was approved by the Ethics Committee of the University Medical Center, Freiburg.
Results
Patients
There were 21 (37.5 %) women and 35 (62.5 %) men with a median age of 60 years (range, 39–78 years) (Table 1). In 13 cases, the primary tumor was localized in the left upper lobe; in 18 cases, in the right upper lobe; in 11 cases, in the left lower lobe; and in 10 cases, in the right lower lobe. Two tumors were localized in the middle lobe and three were in a central position. In 31 cases, we performed a lobectomy; in 4 cases, a lobectomy in combination with a chest wall resection was carried out; in 1 case, a pneumonectomy was performed; in 3 cases, an extended pneumonectomy was performed; in 2 cases, a segmentectomy was performed; in 12 cases, a sleeve lobectomy was performed; and in 3 cases, a wedge resection was conducted (Table 2).
Table 1.
Patient characteristics and clinicopathological findings
| Characteristics | Value |
|---|---|
| Total number of patients | 56 |
| Gender | Female 21 |
| Male 35 | |
| Median age | 60 years (range, 39–78 years) |
| Histology | Adenocarcinoma 31 |
| Squamous carcinoma 7 | |
| Large cell carcinoma 18 | |
| Tumor stage | pT1 9 |
| pT2 42 | |
| pT3 5 | |
| Lymph node involvement | Yes 30 |
| No 26 |
Table 2.
Localization of the primary tumor and type of surgical resection in all patients
| Characteristics | Value |
|---|---|
| Total number of patients | 56 |
| Localizationa | LUL 13 |
| RUL 18 | |
| LLL 11 | |
| RLL 10 | |
| ML 2 | |
| CP 3 | |
| Localization of metastasis | Brain 46 |
| Bones 2 | |
| Adrenal gland 4 | |
| Soft tissue 4 | |
| Surgeryb | Lobectomy 31 |
| Lobectomy with chest wall resection 4 | |
| Pneumonectomy 1 | |
| Extended pneumonectomy 3 | |
| Segmentectomy 2 | |
| Sleeve lobectomy 12 | |
| Wedge 3 |
LUL left upper lobe, RUL right upper lobe, LLL left lower lobe, RLL right lower lobe, ML middle lobe, CP central position
aLocalization of primary tumor
bType of surgery for resection of the primary tumor
Histology and Tumor Stage
Pathological staging revealed a tumor stage of pT1 in 9 cases, pT2 in 42 cases, and pT3 in 5 cases. In 30 cases, there was no lymph node involvement, while pN1 was observed in 7 cases, pN2 in 18 cases, and pN3 in 1 single case. Histological analysis revealed a squamous subtype in 7 cases, adenocarcinoma in 31 cases, and large cell carcinoma of the lung in 18 cases. In 55 cases, resection of the tumor was macroscopically and microscopically complete. In only one case, the tumor tissue was not completely macroscopically removed.
Metastasis
Forty-six patients suffered from one isolated metastasis, eight patients had two metastases, and one patient had four metastases. Forty-six patients suffered from brain metastasis, and four patients had adrenal gland metastases. Two patients suffered from isolated bone metastasis (sternum and right femur), and four patients had soft tissue metastasis. In 24 cases, the metastases were only surgically resected; in 13 cases, surgical resection was accompanied by chemotherapy or radiation; and the remaining cases were treated by stereotactic radiosurgery. In all cases, resection was macroscopically and microscopically complete.
Morbidity and Mortality
There were no intraoperative deaths. One patient with a single cranial metastasis died postoperatively because of acute respiratory distress syndrome (ARDS) following primary tumor resection. In four cases, postoperative complications occurred after resection of the metastasis. In three patients with a brain metastasis, a single epileptic event, a cerebral edema, and minor bleeding were recorded. In one patient with an adrenal metastasis, secondary wound healing occurred. After resection of the primary tumor we recorded three cases of tachyarrhythmia and four cases of pneumonia. Two patients suffered from ARDS and three patients had an embolic event.
Survival Time and Influencing Factors
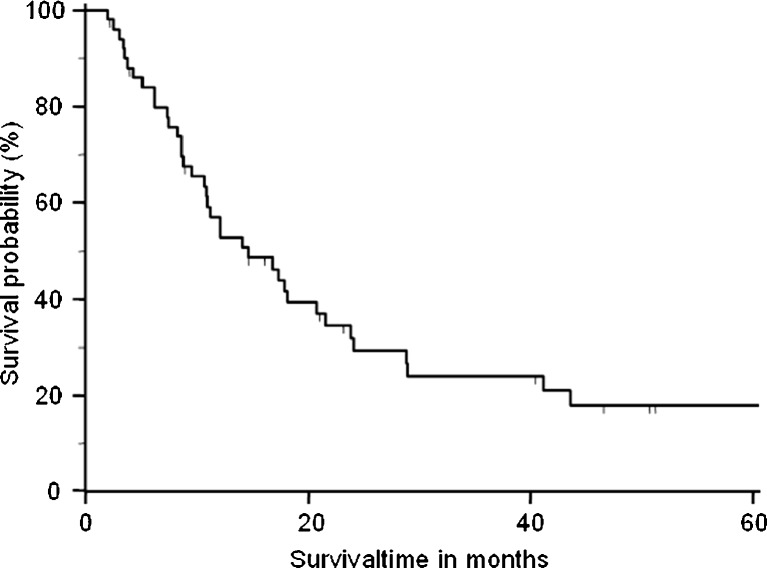
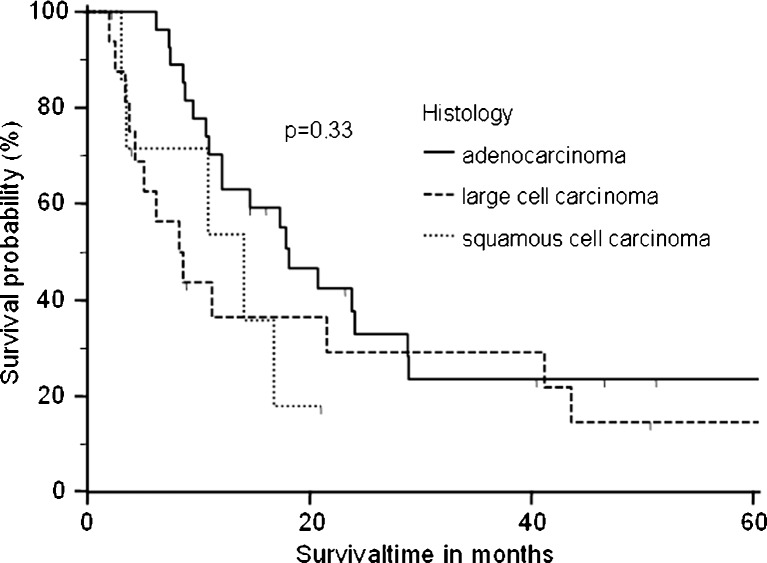
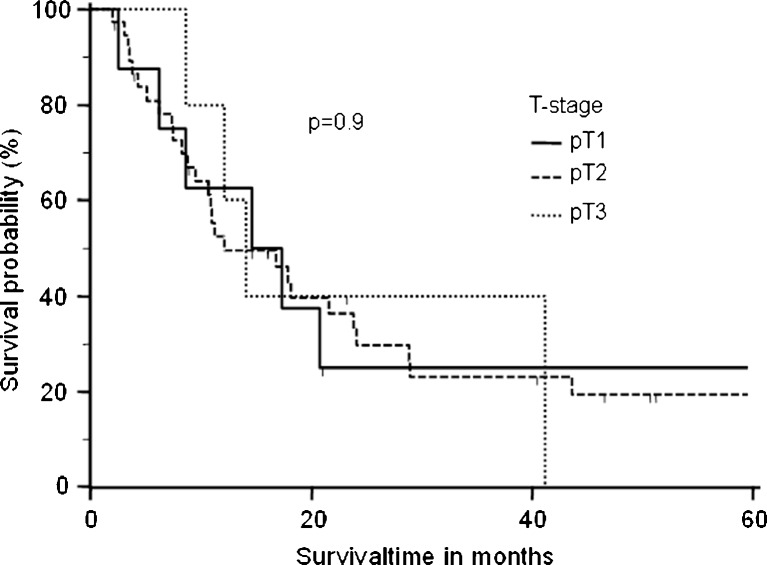
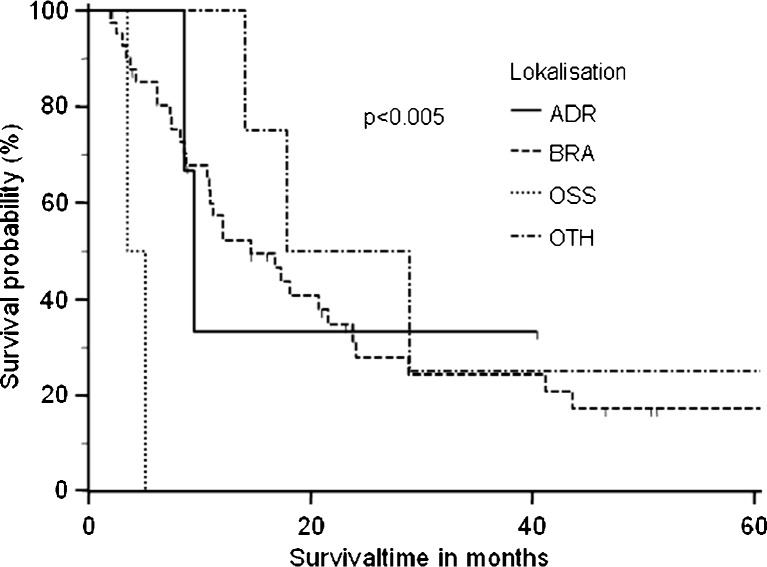
Five patients were lost during follow-up and therefore censored in the survival analysis. One patient died postoperatively because of ARDS. The remaining 50 patients had an overall median survival time of 14.6 months (Fig. 1). Patients with adenocarcinoma had a higher median overall survival time of 18.1 months, than those with squamous cell carcinoma (14.1 months) and large cell carcinoma (8.4 months) (p = 0.33) (Fig. 2). The overall survival was not significantly influenced by tumor stage (pT1, 16.0 months; pT2, 12.1 months; pT3, 14 months; p = 0.9) (Fig. 3). Analyzing the influence of metastatic site, we found a median overall survival of 23.4 months for patients with soft tissue metastasis, 16.7 months for patients with brain metastasis, 9.5 months for patients with adrenal gland involvement, and only 4.3 months for patients with bone metastasis (log-rank test p < 0.005) (Fig. 4). By multivariate analysis using the Cox proportional hazards test, bone metastasis was the only significant parameter [hazard ratio (HR), 11.6; 95 % confidence interval (CI), 2.2–60.5] influencing median overall survival (p < 0.004). Other parameters such as resection margin, type of surgical procedure, localization of primary tumor, tumor grading, or lymph node involvement were not significant by the log-rank or Cox proportional hazards tests.
Fig. 1.
Overall survival time from the time of diagnosis in 50 patients undergoing resection of primary lung cancer. The median overall survival time was 14.6 months
Fig. 2.
Survival time by histological subtype. Median overall survival time differed, although this was not statistically significant
Fig. 3.
Overall survival time according to T stage of the primary lung cancer. The differences did not reach statistical significance
Fig. 4.
The median overall survival differed significantly according to the localization of the metastasis (p < 0.005). Metastasis to the bone was the only significant factor (HR, 11.6; 95 % CI, 2.2–60.5) influencing median overall survival time (p < 0.004)
Discussion
The prognosis of metastatic NSCLC is poor, and platinum-based chemotherapy improves the median survival for only a few months [2]. A subgroup of patients with oligometastatic disease may benefit from surgical resection of the primary tumor and metastasis, but only very limited data are available to date. Several studies have documented a benefit of surgical resection of the primary tumor and a single metastasis in the brain or the adrenal gland, but the benefit of this treatment is not well documented for oligometastatic NSCLC to other metastatic sites [8–10]. Recently, Salah et al. reviewed 51 case reports of patients with oligometastatic, extracranial, and extra-adrenal NSCLC and tried to identify parameters that predict outcome [11]. Overall, the authors reported an encouraging 5-year survival rate of over 50 %. Keeping in mind that Salah et al. used 51 case reports to perform the pooled analysis, it is likely that this high 5-year overall survival rate is at least partially based on a publication bias. Therefore, we analyzed the whole spectrum of oligometastatic NSCLC patients treated in our department. The mean overall survival was 14.6 months, which is much higher than that in patients treated with platinum-based chemotherapy and similar to the median survival reported by others [2, 3, 9, 15]. In line with other authors, we found a different median overall survival depending on histological subgroup, with a better survival for adenocarcinoma (18.1 months in adenocarcinoma vs. 8.6 months in large cell carcinoma vs. 14.1 months in squamous carcinoma), although this was not statistically significant [3]. Regarding T stage, the median survival differed only slightly. Other factors such as resection margins, type of surgical procedure, localization of primary tumor, tumor grading, and lymph node involvement were tested, but none of these parameters had a significant influence on median overall survival. This might be due to the low numbers of patients in the subgroups, which may have concealed the clinical relevance of a single factor. Interestingly, the localization of the metastasis was an important factor influencing the outcome in our cohort. Patients with single metastasis of the bone had a much more unfavorable outcome than others. Although this subgroup was too small to draw any definitive conclusions, it is noteworthy that metastasis of the bone was identified as a significant predictive factor in patients with advanced lung cancer in a different clinical setting [16, 17]. We hypothesize that divergent biological behavior of lung cancer cells exists according to the local micromillieu. Different chemokines and their receptors may influence the organotropy and clinical outcome of lung cancer [18].
The limitations of our study are the small numbers of patients in each subgroup and its retrospective design, which spanned a long collection period. Diagnostic and therapeutic tools have changed dramatically over the years and may have an influence on studies concerning oligometastatic lung cancer. We cannot deny that all of these factors are strongly restricting; however, oligometastatic lung cancer is very rare, and it is extremely difficult to conduct large-scale studies on them. It is known that a small ratio of events per variable can affect the accuracy and precision of regression coefficients and their statistical significance in proportional hazard analysis. Therefore, we failed to identify predictive factors that were statistically significant in our study. However, an interesting observation in our study was the finding that the majority of patients had no or only minimal lymph node involvement. Because we are presenting a nonselected group of patients with metastasis in all locations and the inclusion was based on the same criteria, this might be of some importance in understanding the phenomenon of oligometastatic status. In contrast to the generalized disease of multiple metastatic lung cancer, we hypothesize that oligometastasis is rather a “skip” metastasis in otherwise localized tumors. Further studies are urgently required to investigate the factors predicting the outcome of surgical therapy in patients with oligometastatic lung cancer and the phenomenon of oligometastatic disease.
Acknowledgments
We would like to thank Lydia Faricelli for the excellent assistance and proofreading of this manuscript.
References
- 1.Underwood JM, Townsend JS, Tai E, et al. Racial and regional disparities in lung cancer incidence. Cancer. 2012;118:1910–1918. doi: 10.1002/cncr.26479. [DOI] [PubMed] [Google Scholar]
- 2.Spiro SG, Rudd RM, Souhami RL, et al. Chemotherapy versus supportive care in advanced non-small cell lung cancer: improved survival without detriment to quality of life. Thorax. 2004;59:828–836. doi: 10.1136/thx.2003.020164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Azzoli CG, Baker S, Jr, Temin S, et al. American Society of Clinical Oncology clinical practice guideline update on chemotherapy for stage IV non-small-cell lung cancer. J Clin Oncol. 2009;27:6251–6266. doi: 10.1200/JCO.2009.23.5622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Group N-SCLCC Chemotherapy in non-small cell lung cancer: a meta-analysis using updated data on individual patients from 52 randomised clinical trials. Non-small Cell Lung Cancer Collaborative Group. BMJ. 1995;311:899–909. doi: 10.1136/bmj.311.7010.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Albain KS, Crowley JJ, LeBlanc M, et al. Survival determinants in extensive-stage non-small-cell lung cancer: the Southwest Oncology Group Experience. J Clin Oncol. 1991;9:1618–1626. doi: 10.1200/JCO.1991.9.9.1618. [DOI] [PubMed] [Google Scholar]
- 6.Hellman S, Weichselbaum RR. Oligometastases. J Clin Oncol. 1995;13:8–10. doi: 10.1200/JCO.1995.13.1.8. [DOI] [PubMed] [Google Scholar]
- 7.Bonnette P, Puyo P, Gabriel C, et al. Surgical management of non-small cell lung cancer with synchronous brain metastases. Chest. 2001;119:1469–1475. doi: 10.1378/chest.119.5.1469. [DOI] [PubMed] [Google Scholar]
- 8.Luketich JD, Burt ME. Does resection of adrenal metastases from non-small cell lung cancer improve survival? Ann Thorac Surg. 1996;62:1614–1616. doi: 10.1016/S0003-4975(96)00611-X. [DOI] [PubMed] [Google Scholar]
- 9.Luketich JD, Martini N, Ginsberg RJ, et al. Successful treatment of solitary extracranial metastases from non-small cell lung cancer. Ann Thorac Surg. 1995;60:1609–1611. doi: 10.1016/0003-4975(95)00760-1. [DOI] [PubMed] [Google Scholar]
- 10.Wronski M, Arbit E, Burt M, et al. Survival after surgical treatment of brain metastases from lung cancer: a follow-up study of 231 patients treated between 1976 and 1991. J Neurosurg. 1995;83:605–616. doi: 10.3171/jns.1995.83.4.0605. [DOI] [PubMed] [Google Scholar]
- 11.Salah S, Tanvetyanon T, Abbasi S (2011) Metastatectomy for extra-cranial extra-adrenal non-small cell lung cancer solitary metastases: systematic review and analysis of reported cases. Lung Cancer. doi:10.1016/j.lungcan.2011.07.014 [DOI] [PubMed]
- 12.Hasselle MD, Haraf DJ, Rusthoven KE, et al. Hypofractionated image-guided radiation therapy for patients with limited volume metastatic non-small cell lung cancer. J Thorac Oncol. 2012;7:376–381. doi: 10.1097/JTO.0b013e31824166a5. [DOI] [PubMed] [Google Scholar]
- 13.Groome PA, Bolejack V, Crowley JJ, et al. The IASLC Lung Cancer Staging Project: validation of the proposals for revision of the T, N, and M descriptors and consequent stage groupings in the forthcoming (seventh) edition of the TNM classification of malignant tumours. J Thorac Oncol. 2007;2:694–705. doi: 10.1097/JTO.0b013e31812d05d5. [DOI] [PubMed] [Google Scholar]
- 14.Travis WD TVC, Corrin B (1999) Histological typing of lung and pleural tumours (International Histological Classification of Tumours). Springer, Berlin
- 15.Pfannschmidt J, Dienemann H. Surgical treatment of oligometastatic non-small cell lung cancer. Lung Cancer. 2010;69:251–258. doi: 10.1016/j.lungcan.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 16.Dai L, Fang J, Nie J, et al. Analysis of prognostic factors of 80 advanced NSCLC patients treated with gefitinib for more than 6 months. Zhongguo Fei Ai Za Zhi. 2010;13:1050–1055. doi: 10.3779/j.issn.1009-3419.2010.11.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoang T, Xu R, Schiller JH, et al. Clinical model to predict survival in chemonaive patients with advanced non-small-cell lung cancer treated with third-generation chemotherapy regimens based on eastern cooperative oncology group data. J Clin Oncol. 2005;23:175–183. doi: 10.1200/JCO.2005.04.177. [DOI] [PubMed] [Google Scholar]
- 18.Raynaud CM, Mercier O, Dartevelle P, et al. Expression of chemokine receptor CCR6 as a molecular determinant of adrenal metastatic relapse in patients with primary lung cancer. Clin Lung Cancer. 2010;11:187–191. doi: 10.3816/CLC.2010.n.024. [DOI] [PubMed] [Google Scholar]