Abstract
BACKGROUND
Carotid-femoral PWV (cfPWV) is a well-established measure of central arterial stiffness, while brachial-ankle PWV (baPWV) is being used more frequently in East Asian countries. Few studies have simultaneously characterized the distributions and correlates of segment-specific PWV measures and their associations with cardiovascular risk factors.
METHODS
We evaluated segment-specific PWV (cfPWV, baPWV, and femoral-ankle (faPWV)) in 4,974 older-aged African American and Caucasian adults in the community-based Atherosclerosis Risk in Communities (ARIC) Study using a standardized protocol and the OMRON VP-1000 Plus system. We examined the distribution and multivariable-adjusted correlates of PWV measures by race and sex.
RESULTS
Mean age ranged from 74±5 to 76±5 years across race–sex groups. In all race–sex groups, cfPWV correlated with baPWV but not with faPWV, and cfPWV and baPWV were higher with age, whereas faPWV was not. Heart rate and systolic blood pressure (SBP) were positively associated and weight was negatively associated with all PWV measures; however, the associations with age, glycated hemoglobin, triglycerides, and high-density lipoprotein (HDL) cholesterol varied by segment and race–sex group.
CONCLUSIONS
Our findings indicate that cfPWV and faPWV reflect distinct aspects of segment-specific vascular stiffness and their associated profile of cardiovascular risk factors. Even among older adults, age is associated with higher cfPWV and baPWV, but not with faPWV. Understanding factors that ostensibly play a role in increasing arterial stiffness in different arterial territories can inform opportunities for cardiovascular disease (CVD) prevention and risk management.
Keywords: arterial stiffness, arteriosclerosis, atherosclerosis, blood pressure, cardiovascular disease risk factors, elastic artery, epidemiology, hypertension, muscular artery, subclinical cardiovascular disease, vascular stiffness.
Pulse wave velocity (PWV) is a valid and reliable measure of arterial stiffness that predicts cardiovascular disease (CVD) events and all-cause mortality in clinical and community-based studies.1 Carotid-femoral PWV (cfPWV) is the reference standard measurement of central aortic stiffness,2,3 although the use of brachial-ankle PWV (baPWV) is favored in research studies for practical reasons, especially in East Asian countries such as Japan. baPWV can be measured using an automated measurement technique and represents a composite measure of central and peripheral arterial stiffness.4–6
While baPWV is correlated with cardiovascular risk factors7,8 and mortality,9,10 it is unclear how much of the reported associations correspond to the central or peripheral vasculature. Few studies have documented that baPWV is correlated with cfPWV,4,5,11 and peripheral arterial stiffness measured by femoral-ankle PWV (faPWV);8 however, studies have shown conflicting associations between CVD risk factors and faPWV.6,12,13 Age, for example, an established risk factor for arterial stiffening, is not associated with peripheral stiffness,14,15 but is associated with higher cfPWV.16 The reported association between age and baPWV is inconsistent,2,17 suggesting that risk factor associations with baPWV reflect its properties as a composite measure of central and peripheral arterial segments.
Central arteries contain multiple layers of elastin, whereas peripheral arteries contain more smooth muscle cells. It is necessary, therefore, to characterize segment-specific PWV since arterial architecture and function vary considerably across the arterial tree, and arterial tissue remodeling associated with aging and vascular risk factors also varies across arterial territories.18–23
Few studies measure peripheral and central PWV, limiting our knowledge of the degree to which risk factor associations differ by central (cfPWV), peripheral (faPWV), and composite central and peripheral (baPWV) arterial stiffness. Our aim was to measure segment-specific arterial stiffness and evaluate its correlates in a well-characterized population of older adult African American and Caucasian men and women from the Atherosclerosis Risk in Communities (ARIC) Study. Understanding these relationships would inform hypotheses regarding the pathophysiological implications of risk factors for vascular stiffness and the development of CVD.
METHODS
Study population
The ARIC Study is a population-based, longitudinal study of 15,792 participants aged 45–64 years enrolled between 1987 and 1989 in 4 US communities (Forsyth County, North Carolina; Jackson, Mississippi; Minneapolis, Minnesota; and Washington County, Maryland). Details of the baseline visit have been previously described.24 This analysis includes 5,683 participants who attended visit 5 between 2011 and 2013 and had PWV measured (6,538 total participants at visit 5; 65% response rate from 10,036 eligible participants). We excluded participants with missing information on PWV, body mass index (BMI) ≥40kg/m2, major arrhythmias (Minnesota code 8-1-3, 8-3-1, and 8-3-2), Minnesota code 8-1-2 with evidence of biased PWV waveforms, aortic aneurysms, abdominal aorta ≥5cm, history of aortic or peripheral revascularization or aortic graft, aortic stenosis, moderate or greater aortic regurgitation, and missing covariates of interest (BMI, systolic blood pressure (SBP), heart rate, and smoking). Participants who self-identified as Asian and African American participants from Minnesota and Maryland sites were also excluded due to small numbers. After exclusions, the final analytic sample included 4,974 participants. Participants provided written informed consent, and the study was approved by the Institutional Review Boards at all field centers, coordinating center, and central labs and reading centers.
Participants were asked to bring all prescription and nonprescription medications taken within 2 weeks, to not consume food or drinks, and refrain from tobacco and vigorous physical activity after midnight prior to the clinic visit or for 8 hours prior to the visit. Examinees underwent a blood draw, B-mode scan of the abdominal aorta, standard 12-lead electrocardiogram, anthropometric measurements, and interviewer-administered questionnaires to obtain medical history and lifestyle information. Body weight was measured to the nearest 0.1kg, and height was recorded to the nearest centimeter. Waist circumference was measured in duplicate using standardized reference points. Three seated blood pressure measurements were obtained after a 5-minute rest using an oscillometric automated sphygmomanometer (Omron HEM-907 XL, Omron, Kyoto, Japan), and the average of the last 2 measurements was used.
Hypertension was defined as SBP ≥140mm Hg, diastolic blood pressure ≥90mm Hg, or antihypertensive medication use. Diabetes was defined as fasting glucose ≥126mg/dl, non-fasting glucose ≥200mg/dl, antidiabetic medication use, or self-reported diagnosis of diabetes. Prevalent coronary heart disease and stroke were defined by ARIC cohort surveillance through 30 August 2013. Prevalent heart failure was defined as physician reported heart failure or a hospitalization discharge with an ICD code 428.x in the first position prior to visit 5. Prevalent peripheral arterial disease was defined as an ankle-brachial index of <0.9. Standard resting 12-lead electrocardiograms were digitally acquired using a GE MAC 1200 electrocardiograph (GE, Milwaukee, WI) at 10mm/mV calibration and a speed of 25mm/s. Electrocardiograms were centrally processed using GE 12-SL Marquette Version 2001 (GE) at the Epidemiological Cardiology Research Center at the Wake Forest School of Medicine.
Blood samples were obtained following a standardized venipuncture protocol and shipped weekly to ARIC central laboratories where assays for total cholesterol, high-density lipoprotein (HDL) cholesterol, triglycerides, and fasting glucose were performed. Low-density lipoprotein was calculated using the Friedewald equation.25 Glycated hemoglobin (HbA1c) was measured in EDTA whole blood on the Tosoh HPLC Glycohemoglobin Analyzer (Tosoh Medics, San Francisco, CA) using an automated high performance liquid chromatography method calibrated with standard values derived by the National Glycohemoglobin Standardization Program. The laboratory coefficient of variation was 1.9%.
Pulse wave velocity measures
Technicians measured cfPWV, baPWV, and faPWV following a standardized protocol with the automated waveform analyzer VP-1000 Plus (Omron, Kyoto, Japan)26 after participants were supine for 5–10 minutes. Carotid and femoral arterial pressure waveforms were acquired for 30 seconds by applanation tonometry sensors attached on the left common carotid artery (via neck collar) and left common femoral artery (via elastic tape around the hip). Bilateral brachial and posterior-tibial arterial pressure waveforms were detected over 10 seconds by extremities cuffs connected to a plethysmographic and an oscillometric pressure sensor wrapped on both arms and ankles.
PWV was estimated as the distance between 2 arterial recording sites divided by transit time. Distance for cfPWV was measured with a segmometer for PWV measurements (Rosscraft, Surray, Canada), and calculated as the carotid to femoral distance minus the suprasternal notch to carotid distance. Distances for baPWV and faPWV were automatically calculated by the VP-1000 Plus using height-based formulas, as previously described.5 A minimum of 2 measurements were taken per participant and the last 2 measurements were averaged. We included the right baPWV and faPWV measurements for this analysis. Outliers defined as PWV values 3 SDs above or below the mean were excluded from analyses.
Quality assurance for PWV included central training and recertification, quarterly equipment calibration, and ongoing quality control reviews by one of the authors (H.T.) on a stratified random sample of 40 records per month with feedback provided to technicians. Approximately 78% of records were considered optimal quality, 17% were good quality, 3% were acceptable, and none were poor or unacceptable. Repeat visits were conducted for a subset of participants at each field center approximately 4–8 weeks later (n = 79; mean age 75.7 years; 46 females). The intra-class correlation coefficients and 95% confidence intervals were 0.70 (0.59, 0.81) for cfPWV, 0.84 (0.78, 0.90) for baPWV, and 0.69 (0.59, 0.79) for faPWV.27
Statistical methods
Participant characteristics were estimated as means and SDs, medians and 25th and 75th percentiles, or frequencies and percent, where appropriate. The distribution of cfPWV, baPWV, and faPWV was described using cumulative frequency plots by race and sex. Differences between race and between sex within race groups were assessed using the Kolmogorov-Smirnov test. The relationship between PWV measurements and age were evaluated using Spearman correlations. We calculated means and the 95% confidence intervals for cfPWV, baPWV, and faPWV by 5-year age groups stratified by race and sex.
Associations between participant characteristics and risk factors with PWV were evaluated using multivariable linear regression. Independent variables included race, sex, age, center, current smoking, and prevalent coronary heart disease, heart failure, stroke, and peripheral arterial disease. Subsequent models included factors known to be associated with PWV, such as height, BMI, waist circumference, SBP, diastolic blood pressure, heart rate, total cholesterol, HDL, low-density lipoprotein, hypertension, diabetes, and medication use (β-blockers, α-blockers, calcium channel blockers, diuretics, angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers). We retained variables significantly associated with PWV (P < 0.1). Sets of similar factors were evaluated using the model adjusted R 2 values, and those with the largest R 2 were retained in the model. The sets evaluated include the following: (i) diabetes, fasting glucose, and HbA1c, (ii) SBP, mean arterial pressure, pulse pressure, and hypertension, and (iii) BMI, waist circumference, height, and body weight. We report β coefficient estimates, their precision, and the R 2 values for the models. We evaluated nonlinear relationships between PWV and age and investigated first-order interactions between race and sex and with hypertension, diabetes, SBP, overweight (BMI ≥ 25kg/m2), and age. Results are stratified by race and sex because of significant race–sex interactions for all PWV measures. P-values were two-sided with statistical significance of P < 0.05 (SAS, version 9.2, SAS Institute, Cary, NC).
RESULTS
A majority of participants were women (58.6%). The mean age ranged from 74.4±4.9 to 75.8±5.1 years, and the mean BMI ranged from 27.3±4.7 to 29.8±4.9kg/m2 across race–sex groups (Table 1). The proportion of participants who reported current smoking ranged from 6% to 8%, and Caucasians had higher educational attainment compared with their African American counterparts. The prevalence of diabetes ranged from 24% among Caucasian women to 43% among African American men. The prevalence of hypertension ranged from 69% among Caucasian women to 89% among African American women.
Table 1.
Descriptive characteristics of ARIC visit 5 participants with PWV by race and sex
| Caucasian | African American | |||
|---|---|---|---|---|
| Men n = 1,694 | Women n = 2,208 | Men n = 363 | Women n = 709 | |
| Mean ± SD or median (25th, 75th percentile) | Mean ± SD or median (25th, 75th percentile) | Mean ± SD or median (25th, 75th percentile) | Mean ± SD or median (25th, 75th percentile) | |
| Age (years) | 75.8±5.1 | 75.3±5.0 | 74.4±4.9 | 74.5±5.0 |
| Field center, n (%) | ||||
| Forsyth Co., NC | 427 (25.2) | 540 (24.5) | 25 (6.9) | 40 (5.6) |
| Jackson, MS | 0 | 0 | 338 (93.1) | 669 (94.4) |
| Minneapolis, MN | 691 (40.8) | 880 (39.9) | 0 | 0 |
| Washington Co., MD | 576 (34.0) | 788 (35.7) | 0 | 0 |
| Education, n (%) | ||||
| <High school | 146 (8.6) | 199 (9.0) | 108 (29.8) | 186 (26.3) |
| High school | 498 (29.5) | 932 (42.2) | 63 (17.4) | 167 (23.6) |
| >High school | 1,045 (61.9) | 1,076 (48.8) | 192 (52.9) | 355 (50.1) |
| Current smoker, n (%) | 94 (5.6) | 123 (5.6) | 29 (8.0) | 44 (6.2) |
| Height (cm) | 173.9±6.6 | 159.3±6.0 | 174.6±6.8 | 161.4±6.0 |
| Weight (kg) | 85.3±13.8 | 69.2±12.9 | 85.7±14.8 | 77.8±14.0 |
| BMI (kg/m2) | 28.2±4.0 | 27.3±4.7 | 28.1±4.5 | 29.8±4.9 |
| Waist circumference (cm) | 104.1±10.6 | 97.1±13.0 | 100.0±11.5 | 96.7±12.5 |
| SBP (mm Hg) | 127.9±16.5 | 129.9±17.8 | 131.8±17.8 | 135.0±18.4 |
| DBP (mm Hg) | 65.8±10.4 | 65.3±10.2 | 70.4±10.8 | 69.7±10.1 |
| Pulse pressure (mm Hg) | 62.0±13.1 | 64.6±14.7 | 61.4±13.6 | 65.3±15.3 |
| Mean arterial pressure (mm Hg) | 86.5±11.1 | 86.9±11.3 | 90.9±11.9 | 91.5±11.3 |
| Heart rate (bpm) | 59.7±9.7 | 62.8±9.5 | 64.3±10.7 | 63.7±10.7 |
| Hypertension, n (%) | 1,155 (69.2) | 1,505 (68.5) | 294 (81.2) | 630 (89.2) |
| HbA1c (%) | 5.7 (5.4, 6.1) | 5.7 (5.4, 5.9) | 6.0 (5.6, 6.5) | 6.0 (5.6, 6.4) |
| Fasting glucose (mmol/l) | 6.0 (5.6, 6.8) | 5.8 (5.3, 6.4) | 5.9 (5.4, 6.8) | 5.8 (5.3, 6.7) |
| Diabetes, n (%) | 537 (31.9) | 525 (24.0) | 154 (43.0) | 276 (39.3) |
| LDL (mmol/l) | 2.4 (1.9, 3.0) | 2.8 (2.2, 3.4) | 2.5 (2.1, 3.2) | 2.8 (2.3, 3.5) |
| HDL (mmol/l) | 1.1 (1.0, 1.3) | 1.4 (1.2, 1.7) | 1.2 (1.0, 1.4) | 1.4 (1.2, 1.7) |
| Triglycerides (mmol/l) | 1.3 (0.9, 1.8) | 1.3 (1.0, 1.8) | 1.0 (0.8, 1.4) | 1.1 (0.9, 1.4) |
| Prevalent coronary heart disease, n (%) | 401 (24.0) | 166 (7.7) | 44 (12.3) | 55 (7.9) |
| Prevalent heart failure, n (%) | 72 (4.3) | 61 (2.8) | 24 (6.6) | 29 (4.1) |
| Prevalent stroke, n (%) | 52 (3.1) | 44 (2.0) | 21 (5.8) | 31 (4.4) |
| Prevalent peripheral arterial disease (ABI <0.9), n (%) | 36 (2.2) | 29 (1.3) | 23 (6.5) | 40 (5.7) |
| Medication use, n (%) | ||||
| β-Blocker | 532 (32.0) | 637 (29.2) | 81 (23.1) | 155 (22.2) |
| α-Blocker | 59 (3.5) | 49 (2.2) | 17 (4.8) | 43 (6.2) |
| Diuretic | 505 (30.3) | 792 (36.3) | 185 (52.7) | 441 (63.1) |
| Angiotensin-converting enzyme inhibitor | 585 (35.1) | 529 (24.2) | 152 (43.3) | 233 (33.3) |
| Angiotensin receptor blocker | 221 (13.3) | 345 (15.8) | 64 (18.2) | 185 (26.5) |
| Calcium channel blocker | 345 (20.7) | 431 (19.7) | 134 (38.2) | 302 (43.2) |
| Pulse wave velocity | ||||
| cfPWV (cm/s) | 1,162.8±296.9 | 1,120.7±282.3 | 1,247.3±343.7 | 1,222.1±331.5 |
| baPWV (cm/s) | 1,742.1±311.5 | 1,735.5±316.3 | 1,709.4±341.8 | 1,724.3±304.1 |
| faPWV (cm/s) | 1,106.1±1788.8 | 1,114.3±178.0 | 1,049.0±187.2 | 1,051.8±176.0 |
Abbreviations: ABI, ankle-brachial index; baPWV, brachial-ankle pulse wave velocity; BMI, body mass index; Co., county; cfPWV, carotid-femoral pulse wave velocity; DBP, diastolic blood pressure; faPWV, femoral-ankle pulse wave velocity; HbA1c, glycated hemoglobin; HDL, high-density lipoprotein; LDL, low-density lipoprotein; MD, Maryland; MN, Minnesota; MS, Mississippi; NC, North Carolina; PWV, pulse wave velocity; SBP, systolic blood pressure.
The distribution of PWV varied across race–sex groups. cfPWV was higher among African Americans compared with Caucasians and among Caucasian men compared with Caucasian women, as shown by the cumulative distribution curves (P < 0.001 for race, P = 0.001 for sex within Caucasians, P = 0.07 for sex within African Americans; Supplementary Figure 1). The cumulative distribution curves of baPWV did not vary significantly by race–sex group (P = 0.11 for race, P = 0.41 for sex within Caucasians, P = 0.19 for sex within African Americans; Supplementary Figure 2). faPWV was higher among Caucasians than African Americans, but no difference was seen by sex (P < 0.001 for race, P = 0.39 for sex within Caucasians, P = 0.91 for sex within African Americans; Supplementary Figure 3).
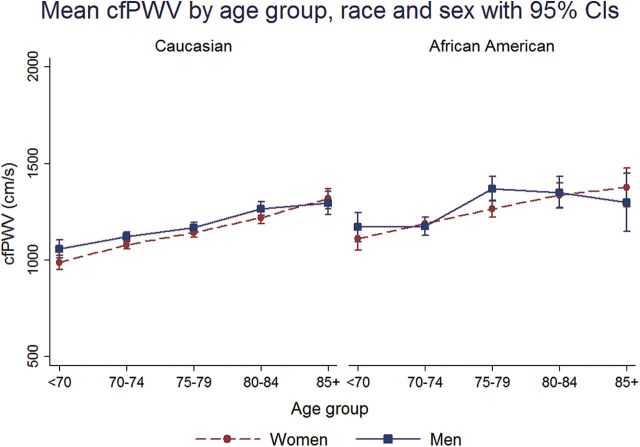
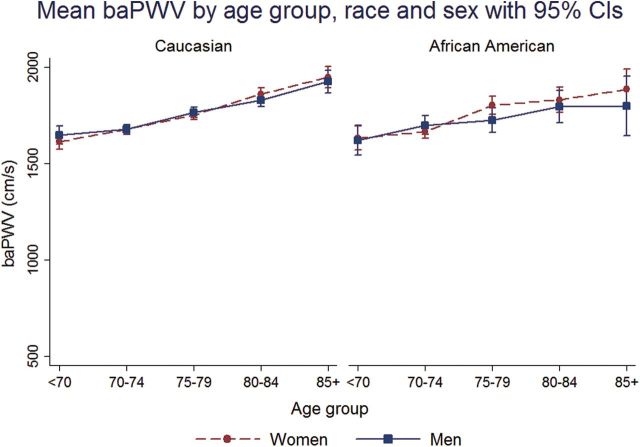
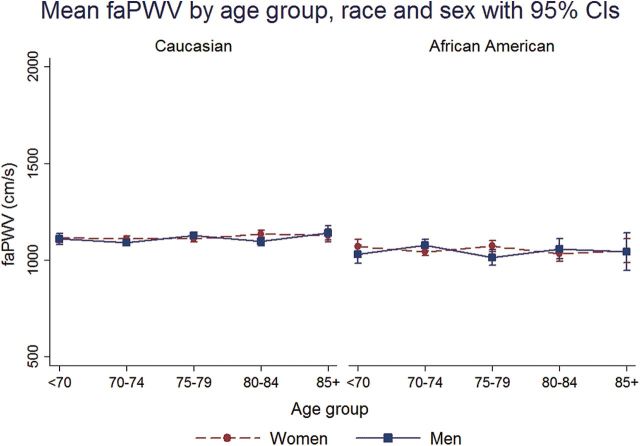
In all race–sex groups, mean cfPWV and baPWV was higher across 5-year age groups from <70 to 85+ years (Figures 1 and 2). faPWV did not differ across age groups (Figure 3). In all race–sex groups, age was positively correlated with cfPWV and baPWV (Spearman correlation range: 0.17–0.30), but not with faPWV (Spearman correlation range: −0.04 to 0.04; Table 2). cfPWV was positively correlated with baPWV in all race–sex groups (Spearman correlation range: 0.40–0.56), and only faPWV correlated with baPWV in all race–sex groups (Spearman correlation range: 0.58–0.70; Table 2).
Figure 1.
Mean carotid-femoral pulse wave velocity (cfPWV) by age group, race, and sex with 95% confidence intervals (CIs).
Figure 2.
Mean brachial-ankle pulse wave velocity (baPWV) by age group, race, and sex with 95% confidence intervals (CIs).
Figure 3.
Mean femoral-ankle pulse wave velocity (faPWV) by age group, race, and sex with 95% confidence intervals (CIs).
Table 2.
Spearman correlations with PWV by race and sex, values are correlation coefficient and P-value
| Caucasian | African American | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Men (n = 1,464–1,660) | Women (n = 1,973–2,023) | Men (n = 334–359) | Women (n = 657–693) | |||||||||
| cfPWV | baPWV | faPWV | cfPWV | baPWV | faPWV | cfPWV | baPWV | faPWV | cfPWV | baPWV | faPWV | |
| Age | 0.23 | 0.27 | 0.04 | 0.3 | 0.29 | 0.04 | 0.25 | 0.17 | −0.04 | 0.22 | 0.25 | −0.01 |
| <0.0001 | <0.0001 | 0.11 | <0.0001 | <0.0001 | 0.07 | <0.0001 | 0.001 | 0.47 | <0.0001 | <0.0001 | 0.78 | |
| cfPWV | 0.52 | 0.02 | 0.56 | 0.02 | 0.4 | −0.05 | 0.41 | −0.06 | ||||
| <0.0001 | 0.41 | <0.0001 | 0.46 | <0.0001 | 0.39 | <0.0001 | 0.12 | |||||
| baPWV | 0.61 | 0.58 | 0.7 | 0.6 | ||||||||
| <0.0001 | <0.0001 | <0.0001 | <0.0001 | |||||||||
Abbreviations: baPWV, brachial-ankle pulse wave velocity; cfPWV, carotid-femoral pulse wave velocity; faPWV, femoral-ankle pulse wave velocity; n, number.
A common set of covariates were significantly related to PWV segments in the stepwise regression analyses (Table 3). Among sets of similar factors, those retained in the model according to R 2 values included HbA1c, SBP, height, and body weight. Age was positively associated with cfPWV and baPWV, whereas age had a weak negative association with faPWV among African American women (Table 3). Heart rate and SBP were positively associated and body weight was negatively associated with all PWV segments.
Table 3.
ARIC visit 5 multivariable linear regression associations with PWV by race and sex
| Caucasian men | Caucasian women | African American men | African American women | |
|---|---|---|---|---|
| Covariate | β (95% CI) | β (95% CI) | β (95% CI) | β (95% CI) |
| cfPWV | n = 1,425, R2 = 0.23 | n = 1,903, R2 = 0.25 | n = 315, R2 = 0.24 | n = 633, R2 = 0.25 |
| Age (years) | 12.4 (9.5, 15.3)§ | 12.7 (10.3, 15.0)§ | 14.9 (7.9, 22.0)§ | 11.5 (6.5, 16.4)§ |
| Heart rate (bpm) | 6.8 (5.3, 8.3)§ | 6.8 (5.6, 8.1)§ | 9.9 (6.5, 13.2)§ | 6.5 (4.3, 8.7)§ |
| SBP (mm Hg) | 4.9 (4.0, 5.7)§ | 4.7 (4.0, 5.3)§ | 5.5 (3.3, 7.7)§ | 5.2 (4.0, 6.5)§ |
| Height (cm) | 3.9 (1.5, 6.4)† | 5.8 (3.7, 7.8)§ | 6.1 (0.4, 11.9)* | 4.9 (0.7, 9.1)* |
| Weight (kg) | −1.4 (−2.8, −0.1)* | −2.5 (−3.5, −1.4)§ | −3.0 (−5.9, −0.2)* | −3.6 (−5.6, −1.6)‡ |
| HbA1c (%) | 52.8 (33.9, 71.6)§ | 41.2 (23.9, 58.5)§ | 34.8 (4.5, 65.2)* | 30.7 (3.9, 57.5)* |
| Triglycerides (mmol/l) | 24.7 (5.2, 44.3)* | 15.7 (−1.1, 32.5) | −12.5 (−68.4, 43.5) | 58.6 (5.1, 112.1)* |
| HDL (mmol/l) | −24.4 (−76.5, 27.6) | −38.0 (−73.3, −2.7)* | 44.0 (−90.4, 178.4) | −67.3 (−137.2, 2.6) |
| baPWV | n = 1,595, R2 = 0.28 | n = 2,057, R2 = 0.32 | n = 332, R2 = 0.32 | n = 661, R2 = 0.31 |
| Age (years) | 12.8 (10.0, 15.5)§ | 11.9 (9.5, 14.3)§ | 8.4 (2.0, 14.7)† | 11.4 (7.1, 15.6)§ |
| Heart rate (bpm) | 5.7 (4.3, 7.1)§ | 6.4 (5.1, 7.6)§ | 5.4 (2.4, 8.4)† | 4.7 (2.9, 6.6)§ |
| SBP (mm Hg) | 6.8 (6.0, 7.6)§ | 6.5 (5.8, 7.1)§ | 7.7 (5.8, 9.6)§ | 5.3 (4.2, 6.4)§ |
| Height (cm) | −1.7 (−4.0, 0.7) | 0.5 (−1.6, 2.7) | −3.5 (−8.6, 1.6) | 0.9 (−2.8, 4.5) |
| Weight (kg) | −2.9 (−4.1, −1.7)§ | −5.2 (−6.3, −4.2)§ | −4.4 (−6.9, −1.9)‡ | −6.6 (−8.3, −5.0)§ |
| HbA1c (%) | 27.7 (10.1, 45.3)† | 16.8 (−0.8, 34.5) | 16.0 (−11.2, 43.2) | 38.7 (16.4, 60.9)‡ |
| Triglycerides (mmol/l) | 20.0 (1.2, 38.9)* | 16.9 (−0.2, 33.9) | 25.0 (−25.7, 75.8) | 54.9 (11.4, 98.4)* |
| HDL (mmol/l) | −17.7 (−67.9, 32.5) | −51.7 (−87.6, −15.7)† | −64.0 (−183.6, 55.5) | −28.7 (−88.6, 31.2) |
| faPWV | n = 1,418, R2 = 0.19 | n = 1,887, R2 = 0.17 | n = 314, R2 = 0.22 | n = 634, R2 = 0.18 |
| Age (years) | −0.01 (−1.8, 1.8) | −1.5 (−3.1, −0.03) | −2.7 (−6.6, 1.1) | −3.0 (−5.7, −0.2)* |
| Heart rate (bpm) | 1.6 (0.7, 2.5)‡ | 2.2 (1.4, 3.0)§ | 1.5 (−0.3, 3.3) | 1.6 (0.4, 2.8)† |
| SBP (mm Hg) | 2.5 (2.0, 3.0)§ | 2.0 (1.6, 2.4)§ | 2.4 (1.2, 3.5)§ | 1.4 (0.7, 2.1)§ |
| Height (cm) | −2.3 (−3.8, −0.7)† | −1.6 (−3.0, −0.3)* | −4.8 (−8.0, −1.6)† | −3.2 (−5.5, −0.9)† |
| Weight (kg) | −2.3 (−3.1, −1.4)§ | −3.2 (−3.9, −2.5)§ | −1.4 (−3.0, 0.1) | −2.6 (−3.7, −1.6)§ |
| HbA1c (%) | −0.9 (−10.6, 12.4) | −0.5 (−12.0, 10.9) | −0.02 (−16.8, 16.8) | 8.4 (−6.0, 22.7) |
| Triglycerides (mmol/l) | 15.3 (3.5, 27.2)* | 3.0 (−8.1, 14.1) | 25.5 (−5.3, 56.3) | 17.2 (−12.3, 46.8) |
| HDL (mmol/l) | −12.6 (−45.2, 20.0) | 10.1 (−13.4, 33.5) | 6.6 (−65.1, 78.3) | 13.6 (−25.2, 52.4) |
Values are βs, 95% confidence intervals (95% CI), and model adjusted R 2; models were additionally adjusted for center, current smoking, prevalent comorbidities (coronary heart disease, heart failure, stroke, peripheral arterial disease), β-blockers, α-blockers, calcium channel blockers, and diuretics.
Abbreviations: baPWV, brachial-ankle pulse wave velocity; cfPWV, carotid-femoral pulse wave velocity; faPWV, femoral-ankle pulse wave velocity; HbA1c, glycated hemoglobin; HDL, high-density lipoprotein; PWV, pulse wave velocity; SBP, systolic blood pressure.
*P < 0.05, † P ≤ 0.01, ‡ P ≤ 0.001, § P ≤ 0.0001.
Associations between height, HbA1c, triglycerides, and HDL with PWV differed across PWV segments and race–sex groups (Table 3). Height was positively associated with cfPWV but negatively associated with faPWV. HbA1c was positively associated with cfPWV and baPWV, but not with faPWV. Triglycerides were positively associated with all PWV measures among Caucasian men and with cfPWV among African American women. HDL was negatively associated with cfPWV and baPWV among Caucasian women.
DISCUSSION
This study is the first to investigate simultaneously characteristics associated with central, peripheral, and composite measures of arterial stiffness among a large, biracial population of older adults. Our findings show cfPWV and baPWV were higher across 5-year age groups from <70 to 85+ years, whereas faPWV did not differ across age groups. Additionally, cfPWV and baPWV were moderately correlated measures of arterial stiffness, and a common set of factors were associated with PWV segments in varying degrees. These factors included age, heart rate, SBP, height, body weight, HbA1c, triglycerides, and HDL. Segment-specific associations were observed with age, height, HbA1c, triglycerides, and HDL. Therefore, our data suggest that factors associated with arterial stiffness vary across arterial territories.
William Osler’s axiom that “man is as old as his arteries” typifies the widespread use of arterial stiffness as a biological marker of aging processes.5,17 In this study of community-dwelling older adults, age was positively associated with cfPWV and baPWV, which was observed across all race–sex groups. In contrast, our data show no association between age and faPWV, with the exception of a small inverse association among African American women. The association between faPWV and age is inconsistent, with studies showing no association between faPWV and age28 or weaker positive associations compared with central measures of arterial stiffness.13,29 These observations are consistent with the expectation that the muscular peripheral vasculature is less affected by age-related vascular changes.14,15
Consistent with prior studies, baPWV was significantly correlated with cfPWV,4–6 as would be expected since the 2 measures are non-orthogonal. Also as expected, baPWV was associated with faPWV, although there was no statistically significant correlation between cfPWV and faPWV. This is in contrast with the study by Choo et al. that showed all segments were significantly correlated with each other among a multiracial population of male adults, including cfPWV and faPWV.6 Our results confirm prior studies showing that baPWV largely reflects stiffness of the central arteries,4,5 although peripheral arterial stiffness contributes to a substantial component of baPWV.
This study showed that heart rate, SBP, and weight were major correlates with all PWV segments. Heart rate and SBP have been consistently associated with cfPWV16,30 and baPWV,6,8 and there is evidence of an association with faPWV.6 Weight was negatively associated will all PWV segments in this analysis. Similar to this study, Choo et al. showed that BMI negatively correlated to faPWV, but positively with other PWV segments.6 Obesity is associated with higher cardiac output and lower peripheral vascular resistance31–33 that could contribute to a lower PWV.
The association between metabolic risk factors, such as HbA1c, triglycerides, and HDL with PWV varied across race–sex groups. Although racial differences in arterial hemodynamics have not been well studied, sex differences have been investigated. Brachial artery diameter increases with age in both men and women, but to a greater extent in women.15 Additionally, there is also evidence that the central waveform differs by sex.34 These observations suggest that the differential structural and functional properties of the vasculature that could affect PWV, such as adaptive remodeling, arterial wave reflection, and smaller arterial diameters and dimensions35,36 may explain the differential associations across race–sex groups.
Central arterial stiffness had a stronger association with HbA1c compared to other markers of glucose metabolism. Similar to these results, a previous study in 5,098 adults reported that cfPWV had a stronger association with HbA1c than with fasting glucose or glucose post 2-hour oral glucose tolerant test.37 An association between HbA1c was also reported in hemodialysis patients with and without diabetes.38 Since HbA1c serves as a marker of long-term glucose exposure, the observed association may index the formation of advanced glycation endproducts and cross-linking in collagen molecules39 leading to arterial stiffening.
We observed an association between HDL and cfPWV and baPWV among Caucasian women. Similarly, HDL was not associated with cfPWV, baPWV, or faPWV among a population of American, Japanese, and Korean men.40 Associations, however, were seen with lipoprotein subclasses and baPWV and faPWV. This suggests that an evaluation of lipoprotein subclasses might elucidate the relationship of lipoproteins and arterial stiffness.
Limitations of our study should be noted. The cross-sectional design precludes the assessment of causality in the observed associations. On occasion, some PWV measurements were not collected due to technical issues, participant factors, and scheduling conflicts. Since the African American members of the ARIC cohort predominantly reside in Jackson, MS, the observed associations may not generalize to African Americans as a demographic group. Similarly, our population consisted of older adults, limiting the generalizability of our findings to younger populations. Distance for cfPWV was measured over the body and may not reflect the actual length of the aorta, and the degree of measurement error could also be affected by the tortuosity of the aorta. Height-based formulas to calculate baPWV and faPWV were validated in a Japanese population and may not be applicable to other race or ethnic groups, which would limit generalizability of this study and technology. The potential bias in length calculations limits the ability to compare baPWV and faPWV across ethnic groups, although it would have a small effect on our conclusions since risk factor associations with PWV measures were race and sex stratified.
This study extends the observation that cfPWV and baPWV are correlated and non-orthogonal measures of arterial stiffness, and shows that faPWV has dissimilar associations with risk factors. Despite the correlated nature of cfPWV and baPWV, the latter spans a more heterogeneous arterial tree and subsumes greater heterogeneity. It is thus likely that associations of risk factors with baPWV reflect a composite of the different associations exhibited by some of these factors with cfPWV and with faPWV, while other factors were related comparably to cfPWV and baPWV.
Our results expand on previously reported differences in the profile of factors associated with central (cf) and peripheral (fa) PWV and highlight the benefits of segment-specific measures of PWV. The widely used baPWV predominantly reflects not only the central arterial stiffness but also the peripheral stiffness. Segment-specific measures open the opportunity to understand factors that ostensibly play a role in increasing arterial stiffness in different arterial territories and can inform opportunities for CVD prevention and risk management.
SUPPLEMENTARY MATERIAL
Supplementary materials are available at American Journal of Hypertension (http://ajh.oxfordjournals.org).
DISCLOSURE
The authors declared no conflict of interest.
Supplementary Material
ACKNOWLEDGMENTS
The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN2682 01100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C). We thank the staff and participants of the ARIC study for their important contributions. M.L.M. was supported by the NHLBI T32 training grant HL-007055. S.C. was supported by the Ellison Foundation and NHLBI grant R00HL107642.
REFERENCES
- 1. Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. J Am Coll Cardiol 2010; 55:1318–1327. [DOI] [PubMed] [Google Scholar]
- 2. O'Rourke MF, Staessen JA, Vlachopoulos C, Duprez D, Plante GE. Clinical applications of arterial stiffness; definitions and reference values. Am J Hypertens 2002; 15:426–444. [DOI] [PubMed] [Google Scholar]
- 3. Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, Pannier B, Vlachopoulos C, Wilkinson I, Struijker-Boudier H; European Network for Non-invasive Investigation of Large Arteries. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J 2006; 27:2588–2605. [DOI] [PubMed] [Google Scholar]
- 4. Sugawara J, Hayashi K, Yokoi T, Cortez-Cooper MY, DeVan AE, Anton MA, Tanaka H. Brachial-ankle pulse wave velocity: an index of central arterial stiffness? J Hum Hypertens 2005; 19:401–406. [DOI] [PubMed] [Google Scholar]
- 5. Tanaka H, Munakata M, Kawano Y, Ohishi M, Shoji T, Sugawara J, Tomiyama H, Yamashina A, Yasuda H, Sawayama T, Ozawa T. Comparison between carotid-femoral and brachial-ankle pulse wave velocity as measures of arterial stiffness. J Hypertens 2009; 27:2022–2027. [DOI] [PubMed] [Google Scholar]
- 6. Choo J, Shin C, Barinas-Mitchell E, Masaki K, Willcox BJ, Seto TB, Ueshima H, Lee S, Miura K, Venkitachalam L, Mackey RH, Evans RW, Kuller LH, Sutton-Tyrrell K, Sekikawa A. Regional pulse wave velocities and their cardiovascular risk factors among healthy middle-aged men: a cross-sectional population-based study. BMC Cardiovasc Disord 2014; 14:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hung CS, Lin JW, Hsu CN, Chen HM, Tsai RY, Chien YF, Hwang JJ. Using brachial-ankle pulse wave velocity to associate arterial stiffness with cardiovascular risks. Nutr Metab Cardiovasc Dis 2009; 19:241–246. [DOI] [PubMed] [Google Scholar]
- 8. Tomiyama H, Yamashina A, Arai T, Hirose K, Koji Y, Chikamori T, Hori S, Yamamoto Y, Doba N, Hinohara S. Influences of age and gender on results of noninvasive brachial-ankle pulse wave velocity measurement–a survey of 12517 subjects. Atherosclerosis 2003; 166:303–309. [DOI] [PubMed] [Google Scholar]
- 9. Matsuoka O, Otsuka K, Murakami S, Hotta N, Yamanaka G, Kubo Y, Yamanaka T, Shinagawa M, Nunoda S, Nishimura Y, Shibata K, Saitoh H, Nishinaga M, Ishine M, Wada T, Okumiya K, Matsubayashi K, Yano S, Ichihara K, Cornélissen G, Halberg F, Ozawa T. Arterial stiffness independently predicts cardiovascular events in an elderly community – Longitudinal Investigation for the Longevity and Aging in Hokkaido County (LILAC) study. Biomed Pharmacother 2005; 59(Suppl 1):S40–S44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sheng CS, Li Y, Li LH, Huang QF, Zeng WF, Kang YY, Zhang L, Liu M, Wei FF, Li GL, Song J, Wang S, Wang JG. Brachial-ankle pulse wave velocity as a predictor of mortality in elderly Chinese. Hypertension 2014; 64:1124–1130. [DOI] [PubMed] [Google Scholar]
- 11. Yu WC, Chuang SY, Lin YP, Chen CH. Brachial-ankle vs carotid-femoral pulse wave velocity as a determinant of cardiovascular structure and function. J Hum Hypertens 2008; 22:24–31. [DOI] [PubMed] [Google Scholar]
- 12. Tillin T, Chambers J, Malik I, Coady E, Byrd S, Mayet J, Wright AR, Kooner J, Shore A, Thom S, Chaturvedi N, Hughes A. Measurement of pulse wave velocity: site matters. J Hypertens 2007; 25:383–389. [DOI] [PubMed] [Google Scholar]
- 13. Tsuchikura S, Shoji T, Kimoto E, Shinohara K, Hatsuda S, Koyama H, Emoto M, Nishizawa Y. Brachial-ankle pulse wave velocity as an index of central arterial stiffness. J Atheroscler Thromb 2010; 17:658–665. [DOI] [PubMed] [Google Scholar]
- 14. Benetos A, Laurent S, Hoeks AP, Boutouyrie PH, Safar ME. Arterial alterations with aging and high blood pressure. A noninvasive study of carotid and femoral arteries. Arterioscler Thromb 1993; 13:90–97. [DOI] [PubMed] [Google Scholar]
- 15. van der Heijden-Spek JJ, Staessen JA, Fagard RH, Hoeks AP, Boudier HA, van Bortel LM. Effect of age on brachial artery wall properties differs from the aorta and is gender dependent: a population study. Hypertension 2000; 35:637–642. [DOI] [PubMed] [Google Scholar]
- 16. Cecelja M, Chowienczyk P. Dissociation of aortic pulse wave velocity with risk factors for cardiovascular disease other than hypertension: a systematic review. Hypertension 2009; 54:1328–1336. [DOI] [PubMed] [Google Scholar]
- 17. Mitchell GF, Parise H, Benjamin EJ, Larson MG, Keyes MJ, Vita JA, Vasan RS, Levy D. Changes in arterial stiffness and wave reflection with advancing age in healthy men and women: the Framingham Heart Study. Hypertension 2004; 43:1239–1245. [DOI] [PubMed] [Google Scholar]
- 18. Boutouyrie P, Laurent S, Benetos A, Girerd XJ, Hoeks AP, Safar ME. Opposing effects of ageing on distal and proximal large arteries in hypertensives. J Hypertens Suppl 1992; 10:S87–S91. [PubMed] [Google Scholar]
- 19. Kawasaki T, Sasayama S, Yagi S, Asakawa T, Hirai T. Non-invasive assessment of the age related changes in stiffness of major branches of the human arteries. Cardiovasc Res 1987; 21:678–687. [DOI] [PubMed] [Google Scholar]
- 20. O'Rourke M. Mechanical principles in arterial disease. Hypertension 1995; 26:2–9. [DOI] [PubMed] [Google Scholar]
- 21. Cavalcante JL, Lima JA, Redheuil A, Al-Mallah MH. Aortic stiffness: current understanding and future directions. J Am Coll Cardiol 2011; 57:1511–1522. [DOI] [PubMed] [Google Scholar]
- 22. Lakatta EG. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part III: cellular and molecular clues to heart and arterial aging. Circulation 2003; 107:490–497. [DOI] [PubMed] [Google Scholar]
- 23. Safar ME. Hypothesis on isolated systolic hypertension in the elderly. J Hum Hypertens 1999; 13:813–815. [DOI] [PubMed] [Google Scholar]
- 24.The ARIC Investigators. The Atherosclerosis Risk in Communities (ARIC) Study: Design and objectives. Am J Epidemiol 1989; 129:687–702. [PubMed] [Google Scholar]
- 25. Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 1972; 18:499–502. [PubMed] [Google Scholar]
- 26. Cortez-Cooper MY, Supak JA, Tanaka H. A new device for automatic measurements of arterial stiffness and ankle-brachial index. Am J Cardiol 2003; 91:1519–1522, A9. [DOI] [PubMed] [Google Scholar]
- 27. Snyder M, Tanaka H, Palta P, Patel M, Camplain R, Couper D, Cheng S, Al Qunaibet A, Poon A, Heiss G. Abstract p183: Repeatability of pulse wave velocity: The atherosclerosis risk in communities (aric) study. Circulation 2015; 131:AP183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ito N, Ohishi M, Takagi T, Terai M, Shiota A, Hayashi N, Rakugi H, Ogihara T. Clinical usefulness and limitations of brachial-ankle pulse wave velocity in the evaluation of cardiovascular complications in hypertensive patients. Hypertens Res 2006; 29:989–995. [DOI] [PubMed] [Google Scholar]
- 29. Kimoto E, Shoji T, Shinohara K, Inaba M, Okuno Y, Miki T, Koyama H, Emoto M, Nishizawa Y. Preferential stiffening of central over peripheral arteries in type 2 diabetes. Diabetes 2003; 52:448–452. [DOI] [PubMed] [Google Scholar]
- 30. Mackey RH, Sutton-Tyrrell K, Vaitkevicius PV, Sakkinen PA, Lyles MF, Spurgeon HA, Lakatta EG, Kuller LH. Correlates of aortic stiffness in elderly individuals: a subgroup of the Cardiovascular Health Study. Am J Hypertens 2002; 15:16–23. [DOI] [PubMed] [Google Scholar]
- 31. Tanaka H, Safar ME. Influence of lifestyle modification on arterial stiffness and wave reflections. Am J Hypertens 2005; 18:137–144. [DOI] [PubMed] [Google Scholar]
- 32. Oren S, Grossman E, Frohlich ED. Arterial and venous compliance in obese and nonobese subjects. Am J Cardiol 1996; 77:665–667. [DOI] [PubMed] [Google Scholar]
- 33. Messerli FH, Sundgaard-Riise K, Reisin E, Dreslinski G, Dunn FG, Frohlich E. Disparate cardiovascular effects of obesity and arterial hypertension. Am J Med 1983; 74:808–812. [DOI] [PubMed] [Google Scholar]
- 34. Hayward CS, Kelly RP. Gender-related differences in the central arterial pressure waveform. J Am Coll Cardiol 1997; 30:1863–1871. [DOI] [PubMed] [Google Scholar]
- 35. Gatzka CD, Kingwell BA, Cameron JD, Berry KL, Liang YL, Dewar EM, Reid CM, Jennings GL, Dart AM, Inhibitor AiACOToA-CE, Diuretic-Based Treatment of Hypertension in the E. Gender differences in the timing of arterial wave reflection beyond differences in body height. J Hypertens 2001; 19:2197–2203. [DOI] [PubMed] [Google Scholar]
- 36. Smulyan H, Asmar RG, Rudnicki A, London GM, Safar ME. Comparative effects of aging in men and women on the properties of the arterial tree. J Am Coll Cardiol 2001; 37:1374–1380. [DOI] [PubMed] [Google Scholar]
- 37. Liang J, Zhou N, Teng F, Zou C, Xue Y, Yang M, Song H, Qi L. Hemoglobin A1c levels and aortic arterial stiffness: the Cardiometabolic Risk in Chinese (CRC) Study. PLoS One 2012; 7:e38485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Matsumae T, Abe Y, Murakami G, Ueda K, Saito T. Effects of glucose metabolism on aortic pulse wave velocity in hemodialysis patients with and without diabetes. Hypertens Res 2008; 31:1365–1372. [DOI] [PubMed] [Google Scholar]
- 39. Zieman SJ, Melenovsky V, Kass DA. Mechanisms, pathophysiology, and therapy of arterial stiffness. Arterioscler Thromb Vasc Biol 2005; 25:932–943. [DOI] [PubMed] [Google Scholar]
- 40. Vishnu A, Choo J, Masaki KH, Mackey RH, Barinas-Mitchell E, Shin C, Willcox BJ, El-Saed A, Seto TB, Fujiyoshi A, Miura K, Lee S, Sutton-Tyrrell K, Kuller LH, Ueshima H, Sekikawa A; ERA JUMP Study Group. Particle numbers of lipoprotein subclasses and arterial stiffness among middle-aged men from the ERA JUMP study. J Hum Hypertens 2014; 28:111–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.