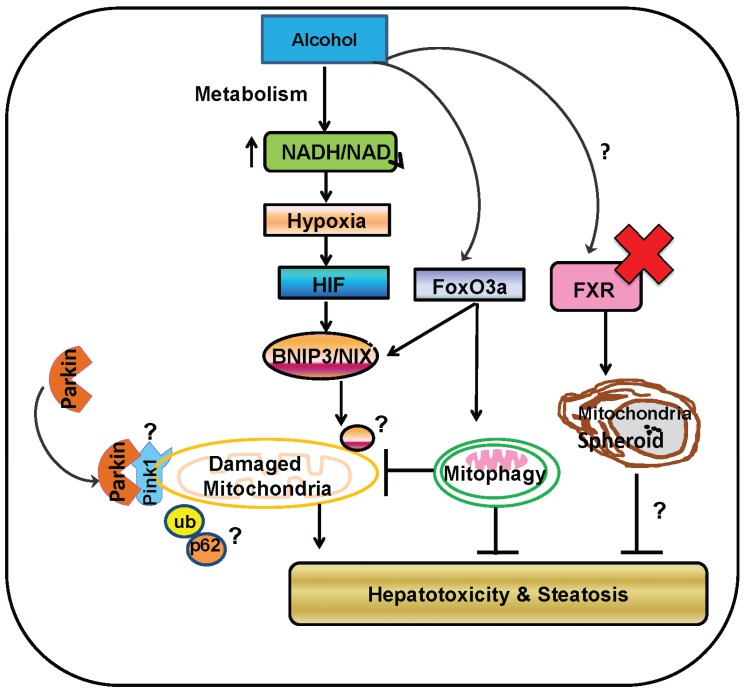
Figure 2.
A proposed model for Parkin-dependent and-independent mitophagy and mitochondrial spheroid formation in alcohol-induced mitochondrial damage and liver injury. In ethanol-exposed hepatocytes, ethanol is metabolized by hepatic enzymes that lead to an increased NADH/NAD+ ratio and subsequent mitochondrial damage. Ethanol induces Parkin translocation from cytosol to a depolarized mitochondrion, which is likely mediated by stabilization of the mitochondrial protein kinase PINK1 on the depolarized mitochondrion. Parkin then ubiquitinates outer mitochondrial membrane proteins, which further recruits autophagy receptor proteins such as p62 to the damaged mitochondrion and triggers selective mitophagy. Meanwhile, ethanol also activates HIF and FoxO3a transcription factors resulting in increased expression of the two atypical BH-3 domain containing proteins BNIP3 and NIX, which might also serve as selective mitophagy receptors by directly interacting with the autophagy protein LC3 to recruit autophagosomes to damaged mitochondria independent of Parkin. In the absence of the nuclear receptor FXR, ethanol also induces formation of mitochondrial spheroids, which may also serve as an alternative mitochondrial quality control pathway to protect against alcohol-induced liver injury. In general, mitophagy protects against alcohol-induced liver injury and steatosis by removing damaged mitochondria and improving adaptive capacity of mitochondria to stress. Modulating Parkin-dependent and-independent mitophagy may be a promising avenue for preventing and treating ALD.