Dear Editor,
Relapses after therapy-induced remissions remain a major challenge in cancer(1, 2). Molecular detection of minimal residual disease (MRD) is valuable in relapse prediction but its cellular basis remains largely unknown(3-5). Delineating therapy-resistant cells (Figure 1A), might inform new therapeutic strategies. ‘Cancer stem cells’, defined by xenografting, have been suggested to preferentially evade therapy but evidence for persistence of phenotypically and functionally distinct cells in patients undergoing treatment is largely lacking.
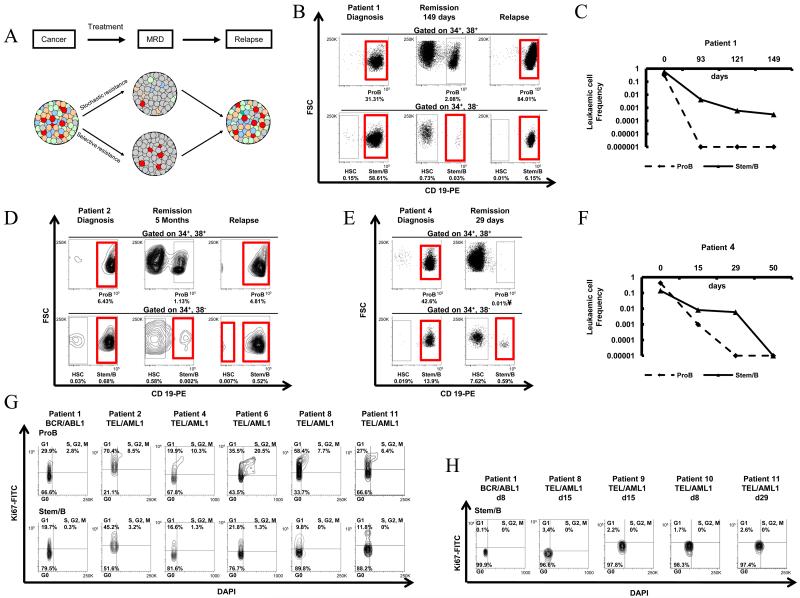
Figure 1. Highly quiescent leukaemic cells account for MRD in cALL.
A. Model depicting cellular bases for stochastic and selective resistance in cALL. B. Differential persistence of Stem/B cells in remission phase of a BCR/ABL1+ cALL case that eventually relapsed; detailed patient characteristics on this and other cALL patients, including MRD results are described in Table 1. FACS analysis of HSC, Stem/B and ProB compartments in diagnostic, remission and relapse samples are shown with the percentage contribution of these populations in total BM MNC indicated. Populations identified as fusion gene positive by FISH, RQ-PCR and/or immunophenotyping are gated in red. Details of fusion-gene analyses in these and related samples are described in Supplemental Table 1. The frequencies of leukaemic ProB and Stem/B cells in this patient relative to total BM MNC (set as 1) at diagnosis, day93, day121 and day149 after initiation of chemotherapy are graphed in panel C. D. Differential persistence of Stem/B cells in remission phase of a TEL-AML+ cALL case that eventually relapsed. FACS analysis of HSCs, ProB cells and Stem/B cells in a TEL/AML1+ cALL case at diagnosis, remission and relapse (for patient characteristics and MRD results, see Table 1). Percentages of HSCs, Stem/B and ProB cells in total BM MNC are indicated. Populations identified as TEL-AML1-positive by FISH, RQ-PCR and/or immunophenotyping are gated in red (see Supplemental Table 1 for details) E. Differential kinetics of ProB and Stem/B cell elimination during initial chemotherapy in a good prognosis TEL-AML1 patient. FACS analysis of cell compartments at diagnosis (left) and day 29 following initiation of chemotherapy (right). Percentages of HSCs, Stem/B and ProB cells in total BM MNC are shown (¥no cells could be sorted) and populations determined as leukaemic by RQ-PCR and/or FISH analysis are gated in red (see Supplemental Table 1 for details). Frequencies of leukaemic ProB and Stem/B cells, relative to total BM MNC (set as 1) at diagnosis, day15, day29 and day50 after initiation of chemotherapy are graphed in F. G. Cell cycle analysis of leukaemic Stem/B and ProB cells at diagnosis in one BCR/ABL1+ and five TEL/AML1+ cALLs. Percentages of cells in G0 (DAPI−Ki67−), G1 (DAPI−Ki67+) and S/G2/M (DAPI+Ki67+) are shown. H. Cell cycle analysis of residual Stem/B cells in MRD samples of one BCR/ABL1+ and 4 TEL/AML1+ cALLs.
We investigated the cells responsible for MRD in childhood acute lymphoblastic leukaemia (cALL) (for patient information see Table 1), where molecular MRD-monitoring has had a practise-changing impact(6). At presentation cALLs comprise phenotypically distinct B-cell stages including ProB-like [CD34+CD38+CD19+], and cells dubbed ‘Stem/B’ co-expressing stem [CD34+CD38−/low] and B-cell [CD19+] markers, seen only in leukaemia and pre-leukaemia(7, 8). Although recent studies suggest that cells capable of propagating cALL in xenografts are plastic and extend beyond the Stem/B compartment(9), its leukaemia-specificity provides a readily trackable biomarker in patients.
Table 1. cALL Patient characteristics.
| Patient | Classification | Translocation | Age at diagnosis | Sex | WBC at diagnosis ×10e9/l | 1. MRD (%) | 2. MRD (%) | 3. MRD (%) | 4. MRD (%) | relapse | SCT | current patient status |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Pre-B ALL | BCR/ABL1 | 7 | F | 110.0 | > 0.01 (day 29)# | 0.002 (day 178)a | < 0.006 (day 239)ab | < 0.001 (day 310)a | 25 months | 8 months | DF post-SCT, 42 months after diagnosis |
| 2 | Pre-B ALL | TEL/AML1 | 3 | F | 120.0 | 0.48 (day 15) | 0.04 (day 33) | 0.05 (day 78) | 0.12 (5 months) | 10 months | no | dead, 13 months after diagnosis |
| 3 | Pre-B ALL | TEL/AML1 | 3 | F | 4.6 | 1.78 (day 8) | 1.25 (day 15) | < 0.001 (day33) | < 0.001 (day98) | 17.5 months | 21.5 months | DF post-SCT, 66 months after diagnosis |
| 4 | Pre-B ALL | TEL/AML1 | 7 | M | 2.5 | 4.1 (day 15) | 0.092 (day 29) | < 0.1 (day 50) | < 0.1 (day 106) | no | no | DF 1st remission, 95 months after diagnosis |
| 5.3 (day 15)* | 0.03 (day 29)* | < 0.1 (day 50)* | 0.013 (day 106)* | |||||||||
| 5 | Pre-B ALL | TEL/AML1 | 5 | M | 39.0 | 2.3 (day 15) | 0.003 (day 29) | 0.0012 (day 50) | < 0.1 (day 106) | no | no | DF 1st remission, 96 months after diagnosis |
| 0.3 (day 15)* | 0.004 (day 29)* | 0.07 (day 50)* | 0.014 (day 106)* | |||||||||
| 6 | Pre-B ALL | TEL/AML1 | 3 | M | 4.1 | n/a | n/a | n/a | n/a | no | no | DF 1st remission, 89 months after diagnosis |
| 0.14 (day 15)* | 0.02 (day 29)* | < 0.1 (day 50)* | < 0.1 (day 106)* | |||||||||
| 7 | Pre-B ALL | TEL/AML1 | 4 | M | 1.8 | > 0.01 (day 29)# | n/a | n/a | n/a | no | no | DF 1st remission, 51 months after diagnosis |
| 8 | Pre-B ALL | TEL/AML1 | 5 | M | 13.0 | 0.19 (day 15) | 0.0012 (day 29) | < 0.1 (day 50) | < 0.1 (day 106) | no | no | DF 1st remission, 89 months after diagnosis |
| 0.1 (day 15)* | 0.01 (day 29)* | < 0.1 (day 50)* | < 0.1 (day 106)* | |||||||||
| 9 | Pre-B ALL | TEL/AML1 | 3 | M | 15.0 | > 0.01 (day 29)# | n/a | n/a | n/a | no | no | DF 1st remission, 13 months after diagnosis |
| 10 | Pre-B ALL | TEL/AML1 | 1 | M | 71.0 | < 0.01 (day 29) | n/a | n/a | n/a | no | no | DF 1st remission, 10 months after diagnosis |
| 11 | Pre-B ALL | TEL/AML1 | 6 | F | 43.0 | > 0.01 (day 29)# | n/a | n/a | n/a | no | no | DF 1st remission, 9 months after diagnosis |
Unless otherwise specified MRD was quantified in aspirated BM samples by polymerase chain reaction of immunoglobulin (IG) and/or T-cell receptor (TCR) rearrangements of at least 1 clonal marker with a sensitivity of at least 10−4 for patient 1, 2, 7, 9, 10 and 11, 10−5 for patient 3 and 10−3 for patient 4, 5 and 8.
MRD was determined by RT-PCR for BCR/ABL1 (p190) and the control gene ABL. Results are displayed as BCR/ABL1:ABL Ratio (%).
MRD was determined from PB.
exact MRD values are not available.
MRD values were measured by flow cytometry with a standardized protocol of triple monoclonal antibody (Mab) combinations and a sensitivity of at least 10−3. WBC, white blood count; MRD, minimal residual disease; SCT, stem cell transplantation; DF, disease free; n/a, not available
We first tracked the fate of leukaemic cells in MRD-positive remission samples of three cALL patients that went on to relapse. In patient 1, BCR/ABL1-positive ‘Stem/B’ cells selectively persisted at remission, when no BCR/ABL1+ leukaemic cells could be found in other B cell compartments (Figure 1B, C). A similar picture emerged from the analysis of ProB-like and Stem/B cells in remission samples in two TEL/AML1 cases (patients 2 and 3; Figure 1D and Supplemental Figure 1A) suggesting that in patients with relapsed disease, cells within the Stem/B compartment are selectively chemoresistant, sustaining MRD and potentially initiating relapse.
In good-prognosis TEL/AML1 patients that achieved and remain in long-term complete (MRD-negative) remission, we observed two behaviours; either Stem/B cells were eliminated with similar kinetics to ProB-like cells (1 patient; Supplemental Figure 1B) or, more slowly than their ProB counterparts (4 patients; Figure 1E, F and Supplemental Figure 1C-E).
Though variable, as might be expected in a setting where all leukaemic cells are ultimately eliminated, the preferential chemoresistance of Stem/B cells seen in most patients suggests enrichment of cells with specific functional properties within the Stem/B compartment. We speculated that the Stem/B phenotype may represent a surrogate, biomarker for quiescence. At diagnosis, Stem/B cells were more quiescent (G0) and less actively cycling (S-M-G2) than leukaemic ProB cells, in all 6 patients analyzed (Figure 1G). Moreover, in all MRD samples investigated we found that chemotherapy further selected for a rare but almost exclusively quiescent (G0) population of Stem/B cells (Figure 1H).
Our findings (i) suggest that quiescence contributes mechanistically to enhanced chemo-resistance within the Stem/B compartment in cALL, a disease arguably derived from B-cell restricted progenitors which in contrast to stem cells normally cycle extensively; (ii) highlight the importance and feasibility of tracking rare but distinct cancer cell populations in patients undergoing therapy; and (iii) provide a conceptual framework for reframing the cancer stem cell debate in terms of patient relevance in the form of MRD-sustaining and relapse-initiating cells.
Our results merit comparison with those reported by Wilson et al. These workers concluded that there was no specific cellular basis for therapy resistance in patients with cALL (10). This conclusion is in line with their view that there is no specific cellular basis for tumour propagation in xenograft models of cALL (9). The conclusion of the Wilson et al. study are limited by that fact it was solely based on immunophenotypic analysis of patient samples, lacking molecular based PCR or FISH analyses affording unambiguous discrimination between leukaemic and non leukaemic cells. Nevertheless, in line with our own results, Wilson et al observed that the stem/B population, still phenotypically detectable in 7/11 MRD+ remission samples, was proportionally spared during therapy relative to other compartments. Wilson et al chose to interpret these results as reflecting a chemotherapy-induced change in cell surface phenotype rather than a selective persistence of a specific cell compartment. We cannot completely rule out that surface phenotypes change in immediate response to chemotherapy but this does not seem a likely explanation for the selective persistence of distinct immunophenotypes throughout extended remission phases. Regardless and crucially, our results extend beyond simple cell surface analysis. We link the Stem/B phenotype to the functional property of cell quiescence. This relationship was observed in diagnostic samples that have not been exposed to treatment thus eliminating any possibility of therapy induced cell surface modulation. We further show that in all investigated cases therapy enriches for quiescent cells demonstrating the functional relevance of the quiescent state which is enriched within the Stem/B compartment. This highlights the fact that, although enriched for quiescence, not all Stem/B cells are quiescent. Similarly not all quiescent cells within the leukaemia are Stem/B cells. It remains to be determined how quiescence and the Stem/B phonotype are regulated and it is plausible that either or both are mutable states influenced by extrinsic cues such as those, resident within the bone marrow or stem cell niche. Such a view would account for stem cell or tumour propagating cell ‘plasticity’ reported in cALL (9). Despite limitations of our study with cases in which we determined leukaemic cells by aberrant phenotype in follow-up samples or could not detect residual ALL cells in samples with evidence for molecular MRD our data clearly suggest that persistent Stem/B cells in follow up samples carry increased risk of relapse.
The need to eliminate highly quiescent cALL cells provides a plausible explanation for the requirement for prolonged (2-3yrs) maintenance therapy to achieve durable clinical remissions in cALL (11, 12). It also provides a plausible explanation for cALL cases in which the relapse clone resembles the presentation leukemic clone up to 8 years between diagnosis and relapse (13).
One possible approach to eradicate resistant and quiescent ALL cells would be to induce cell cycling. Proof of principle of such an approach has been demonstrated. Normal HSCs are usually resistant to treatment with anti-proliferative drugs such as 5-fluoro-uracil. However, after treatment with IFNα to induce cell cycle entry of HSCs, these cells were efficiently killed with 5-fluoro-uracil (14). In a similar vein, recent xenograft studies have shown that dormant AML cells become susceptible to chemotherapy after cell cycle inducing treatment with G-CSF (15). Therefore, further exploration of combinations of cycle-inducing agents with established chemotherapies might prove a profitable avenue for successful elimination of otherwise resistant cALL cells(15).
Supplementary Material
Acknowledgments
Leukaemia and Lymphoma Research (Specialist Programme Grant to TE), EuroCancerStemCell (6th framework EU Specific Targeted Research Projects to TE and SEJ), National Institute for Health Research Biomedical Research Centre Programme, CBRC, UCL, Haematolinne, IGA-MZ NT/12428-5, MSM0021620813 (JZ), a Deutsche Forschungsgemeinschaft fellowship LU1474/1-1 (CL) and a Career Development Program fellowship from the Leukemia and Lymphoma Society, USA (PSW).
Footnotes
Conflict of interest
The authors declare no conflict of interest.
Supplementary Information is available at the Leukemia website.
References
- 1.Pui CH, Robison LL, Look AT. Acute lymphoblastic leukaemia. Lancet. 2008 Mar 22;371(9617):1030–43. doi: 10.1016/S0140-6736(08)60457-2. [DOI] [PubMed] [Google Scholar]
- 2.Moricke A, Zimmermann M, Reiter A, Henze G, Schrauder A, Gadner H, et al. Long-term results of five consecutive trials in childhood acute lymphoblastic leukemia performed by the ALL-BFM study group from 1981 to 2000. Leukemia. 2010 Feb;24(2):265–84. doi: 10.1038/leu.2009.257. [DOI] [PubMed] [Google Scholar]
- 3.Szczepanski T, Orfao A, van der Velden VH, San Miguel JF, van Dongen JJ. Minimal residual disease in leukaemia patients. Lancet Oncol. 2001 Jul;2(7):409–17. doi: 10.1016/s1470-2045(00)00418-6. [DOI] [PubMed] [Google Scholar]
- 4.Flohr T, Schrauder A, Cazzaniga G, Panzer-Grumayer R, van der Velden V, Fischer S, et al. Minimal residual disease-directed risk stratification using real-time quantitative PCR analysis of immunoglobulin and T-cell receptor gene rearrangements in the international multicenter trial AIEOP-BFM ALL 2000 for childhood acute lymphoblastic leukemia. Leukemia. 2008 Apr;22(4):771–82. doi: 10.1038/leu.2008.5. [DOI] [PubMed] [Google Scholar]
- 5.Conter V, Bartram CR, Valsecchi MG, Schrauder A, Panzer-Grumayer R, Moricke A, et al. Molecular response to treatment redefines all prognostic factors in children and adolescents with B-cell precursor acute lymphoblastic leukemia: results in 3184 patients of the AIEOP-BFM ALL 2000 study. Blood. 2010 Apr 22;115(16):3206–14. doi: 10.1182/blood-2009-10-248146. [DOI] [PubMed] [Google Scholar]
- 6.van Dongen JJ, Seriu T, Panzer-Grumayer ER, Biondi A, Pongers-Willemse MJ, Corral L, et al. Prognostic value of minimal residual disease in acute lymphoblastic leukaemia in childhood. Lancet. 1998 Nov 28;352(9142):1731–8. doi: 10.1016/S0140-6736(98)04058-6. [DOI] [PubMed] [Google Scholar]
- 7.Castor A, Nilsson L, Astrand-Grundstrom I, Buitenhuis M, Ramirez C, Anderson K, et al. Distinct patterns of hematopoietic stem cell involvement in acute lymphoblastic leukemia. Nat Med. 2005 Jun;11(6):630–7. doi: 10.1038/nm1253. [DOI] [PubMed] [Google Scholar]
- 8.Hong D, Gupta R, Ancliff P, Atzberger A, Brown J, Soneji S, et al. Initiating and cancer-propagating cells in TEL-AML1-associated childhood leukemia. Science. 2008 Jan 18;319(5861):336–9. doi: 10.1126/science.1150648. [DOI] [PubMed] [Google Scholar]
- 9.le Viseur C, Hotfilder M, Bomken S, Wilson K, Rottgers S, Schrauder A, et al. In childhood acute lymphoblastic leukemia, blasts at different stages of immunophenotypic maturation have stem cell properties. Cancer Cell. 2008 Jul 8;14(1):47–58. doi: 10.1016/j.ccr.2008.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wilson K, Case M, Minto L, Bailey S, Bown N, Jesson J, et al. Flow minimal residual disease monitoring of candidate leukemic stem cells defined by the immunophenotype, CD34+CD38lowCD19+ in B-lineage childhood acute lymphoblastic leukemia. Haematologica. 2010 Apr;95(4):679–83. doi: 10.3324/haematol.2009.011726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ritter J, Creutzig U, Reiter A, Riehm H, Schellong G. Childhood leukemia: cooperative Berlin-Frankfurt-Munster trials in the Federal Republic of Germany. J Cancer Res Clin Oncol. 1990;116(1):100–3. doi: 10.1007/BF01612648. [DOI] [PubMed] [Google Scholar]
- 12.Tubergen DG, Gilchrist GS, O’Brien RT, Coccia PF, Sather HN, Waskerwitz MJ, et al. Improved outcome with delayed intensification for children with acute lymphoblastic leukemia and intermediate presenting features: a Childrens Cancer Group phase III trial. J Clin Oncol. 1993 Mar;11(3):527–37. doi: 10.1200/JCO.1993.11.3.527. [DOI] [PubMed] [Google Scholar]
- 13.van Delft FW, Horsley S, Colman S, Anderson K, Bateman C, Kempski H, et al. Clonal origins of relapse in ETV6-RUNX1 acute lymphoblastic leukemia. Blood. 2011 Jun 9;117(23):6247–54. doi: 10.1182/blood-2010-10-314674. [DOI] [PubMed] [Google Scholar]
- 14.Essers MA, Offner S, Blanco-Bose WE, Waibler Z, Kalinke U, Duchosal MA, et al. IFNalpha activates dormant haematopoietic stem cells in vivo. Nature. 2009 Apr 16;458(7240):904–8. doi: 10.1038/nature07815. [DOI] [PubMed] [Google Scholar]
- 15.Saito Y, Uchida N, Tanaka S, Suzuki N, Tomizawa-Murasawa M, Sone A, et al. Induction of cell cycle entry eliminates human leukemia stem cells in a mouse model of AML. Nat Biotechnol. 2010 Mar;28(3):275–80. doi: 10.1038/nbt.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.