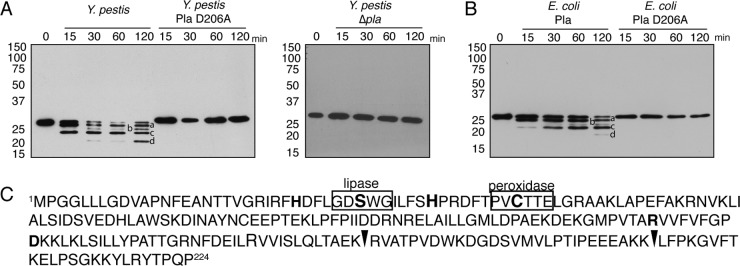
FIG 2.
Cleavage of Prdx6 requires catalytically active Pla. (A) Immunoblot analysis of recombinant human Prdx6 following incubation with Y. pestis, Y. pestis Pla D206A, or Y. pestis Δpla at 37°C for the indicated times. (B) Immunoblot analysis of recombinant human Prdx6 following incubation with E. coli producing Pla or Pla D206A or E. coli carrying the corresponding empty expression vector at 37°C for the indicated times. (C) Amino acid sequence of human Prdx6. Black arrowheads indicate identified Pla cleavage sites. The lipase and peroxidase motifs are boxed, and residues for the active site for peroxidase activity (C47, H39, and R132) or the catalytic triad for lipase activity (S32, D140, and H26) are in bold. Lowercase letters in panels A and B represent Pla-cleaved Prxd6 products. Numbers to the left of the blots indicate molecular masses in kilodaltons. Data are representative of at least 3 independent experiments.