Abstract
The study aims to observe the impacts of thyroxine (T4) combined with donepezil (DON) on hippocampal ultrastructures and expressions of synaptotagmin-1 and SNAP-25 in adult rats with hypothyroidism. All rats were randomly divided into five groups: the normal control group (CON), the hypothyroidism group (Hypo), the T4 treatment group (T4), the DON treatment group (DON) and the T4+DON combined treatment group (T4+DON). Technique of Electron Microscope (TEM) was used to observe the hippocampal ultrastructures of each group, Western blot and real-time RT-PCR were performed to analyze the protein and mRNA expressions of syt-1 and SNAP-25 in the hippocampus of each group. TEM revealed that the Hypo group exhibited the significant vacuolar degeneration of mitochondria in the hippocampal neurons, the free ribosomes were sparse, the synaptic structures were damaged, and the number of synaptic vesicles was reduced, the above injuries in the T4 or DON group were improved, and the performance of the T4+DON group was the most close to the CON group. From the protein and mRNA levels, the dorsal hippocampal syt-1 expression of the Hypo group was significantly reduced, while SNAP-25 was significantly increased, the expressions were partially recovered after the T4 treatment, and the T4+DON combined treatment made the expression return to normal. The adult hypothyroid rats exhibited pathological damages in the hippocampal ultrastructures, the expression of syt-1 was downregulated, while that of SNAP-25 was upregulated, the T4+DON combined therapy could repair the above injuries, and the roles were better than the single drug treatment.
Keywords: Hypothyroidism, hippocampus, ultrastructure, synaptotagmin-1, snap-25, thyroxine, donepezil
Introduction
The thyroid hormones (THs) played an important role in maintaining the normal structures and functions of central nervous system [1]. As the region closely related to cognition, emotion and other functions in the central nervous system, the neurons in the hippocampus are the targets of THs, and there were plenty THs receptors inside [2]. The adult hypothyroidism could cause the damages of morphologies and functions in the hippocampus [3], and the mechanism might be involved in the neurons’ contact and synaptic plasticity, it was a complex physiological process involved by a variety of synaptic proteins. The synapses were the basis for the information transmission among the neurons, the information transmission among the neurons were completed by the exocytotically and quantally released neurotransmitters from presynaptic vesicles. Synaptotagmin-1 (syt-1) and SNAP-25 (synaptosome-associated protein of 25 kDa) were two important synaptic proteins, highly expressed inside the hippocampal neurons. syt-1 was a envelope protein of synaptic vesicle, mainly distributed on the surface of small synaptic vesicles and large dense vesicle in the brain. As the fast Ca2+ receptor, syt-1 could promote the integration of synaptic vesicles, thus playing an important role in regulating the simultaneous release of neurotransmitters [4]. SNAP-25 was a presynaptic plasma membrane protein, involved in the releasing process of Ca+ dependent neurotransmitters, it could combine syntaxin and synaptobrevin to form the core complex of stable SNARE (soluble N-ethylmaleimide-sensitive factor attachment protein receptor), so it was related with the plasticity of synapses [5]. Syt-1 and SNAP-25 could interact with each other, thus jointly involving in the exocytosis and endocytosis process of synaptic vesicles, promoting the release of neurotransmitters, and closely related to learning and memory [6,7]. It had been shown adult hypothyroidism in could lead to the expression changes of such synaptic proteins as syt-1 and SNAP-25 [8].
Currently, the T4 alternative therapy was a conventional means in treating hypothyroidism, which’s treatment standard was to recover the serum thyroid hormone level to normal, but it was still controversial whether it could fully recover the hypothyroidism-caused brain damages. Some studies had shown that the T4 alternative therapy could fully recover the hypothyroidism-caused impairments of cognitive functions and memory [9,10]. Interestingly, certain clinical studies found that even performed the sufficient amount of T4 alternative therapy, some hypothyroid patients still existed the cognitive impairments and depressive symptoms [11-13]. Our previous studies also reported that adult Hypo would affect the expressions of synaptic proteins inside the hippocampus, while after gave regular doses of T4 (5 μg/100 g body weight), and the serum T3 and T4 levels were returned to normal, the damages of synaptic proteins failed to fully recover [8], suggesting that it was necessary to look for other more effective ways to treat hypothyroidism-caused cognitive impairments.
Donepezil (DON) was a cholinesterase inhibitor, through combining with the cholinesterase, it could prevent the hydrolysis of acetylcholine in the brain, thus effectively improving the cognitive and memory impairments [14,15], and it might also have certain therapeutic effects towards hypothyroidism-caused hippocampal damages. In the present study, we investigated the ultrastructures of neurons and synapses in the hippocampus of adult Hypo rats, as well as the expression changes of syt-1 and SNAP-25, aiming to evaluate the impacts of combined T4+DON therapy towards the above outcome indicators.
Materials and methods
Preparation of animal models
60 healthy male SD rats, 3 months old, SPF grade, weighed 230-260 g, were purchased from the Experimental Animal Center of Anhui Medical University (Hefei, China). All rats were given the standard diet, while free to water, the temperature was controlled at 21-23°C, with the humidity as 50±5% and circadian balance. After adaptively fed for one week, the SD rats were randomly divided into five groups: the normal control group (CON), the hypothyroidism group (Hypo), the T4 treatment group (T4), the DON treatment group (DON) and the T4+DON combined treatment group (T4+DON), with 12 rats in each group. The total modeling time was six weeks, the CON group was fed with normal water, while the other four groups were given 0.05% (w/v) propylthiouracil (6-n-propyl-2-thiouracil, PTU; Sigma-Aldrich, St. Louis, MO, USA) every day. 4 weeks later, the T4 group was intraperitoneally injected T4 (Sigma-Aldrich, dissolved in saline, 6 μg/100 g body weight), the DON group added 0.005% DON (Sigma-Aldrich) into the drinking water, and the T4+DON group was performed the above administration simultaneously. Meanwhile, the rest three groups were injected the same amount of saline as the alternative. The total treatment time was two weeks, and the dosage was adjusted according to the rats’ weights weighed weekly.
Sample preparation
After weighed the body weight, the rat was intraperitoneally injected chloral hydrate (0.3 ml/100 g body weight) and opened the abdominal cavity after anesthetized, the abdominal aortic blood was then sampled for the determination of serum T3 and T4 levels. The brain was then sampled on ice after the blood was sampled, the hippocampal tissues of 4 rats of each group were sampled and in 4% paraformaldehyde solution for the TEM observation; the hippocampal tissues of the rest 8 rats of each group were placed at -80°C, the left hippocampus was used for Real-time RT-PCR, while the right hippocampus was performed the Western blot test.
T3 and T4 serum
The radioimmunoassay was performed to test the serum T3 and T4 levels with the radioimmunoassay kit (North Institute of Biological Technology, Beijing, China).
TEM observation
The tissues were cut into small pieces, about 1 mm3, fixed with 2.5% glutaraldehyde at 4°C for 4-6 h, then fixed with l% osmium tetroxide for 1 h, after ethanol dehydration and epoxy resin (Epon812) embedding, the tissues were prepared the ultrathin sections, and soaked in the uranyl acetate solution and the lead citrate solution for the staining, after rinsed conventionally, the ultrastructures were observed and photographed with Nissan JEM-1230 TEM.
Western blot analysis
The total proteins were extracted from the dorsal hippocampus, and prepared as previously described [16]. In brief, the dorsal hippocampal tissues were placed in a glass homogenizer, added RIPA lysis buffer and PMSF, then homogenized on ice to extract the total proteins. The homogenate was then centrifuged at 15000 g and 4°C for 15 min, the supernatant was then determined the total protein concentration with the lorry method: after quantified, added 2× loading buffer (1:1) into the sample, denaturated the proteins at 98°C for 10 min, packaged and cryopreserved at -80°C; 20 ug sample of each group was performed the SDS- polyacrylamide gel electrophoresis for 2.5 h, then transferred onto the membrane for overnight blocking with 5% skim milk. On the following day, the primary antibodies of syt-1 (1:1000, rabbit polyclonal, Abcam, USA), SNAP-25 (1:1000, rabbit polyclonal, Abcam, USA) and GAPDH (1:4 000, rabbit monoclonal, Abcam, USA) were added for 2-h incubation, followed by rinsing with 0.05% PBST solution for three times, 10 min for each time, then the secondary antibody [1:100000, goat-anti-rabbit IgG-horseradish peroxidase (HRP), Abcam, USA] was added and incubated at room temperature for 1-2 h; rinsed the membrane with 0.05% PBST; finally, the enhanced chemiluminescence solution (ECL kit; Pierce Biotechnology, USA) was added and Fine-do X6 visualizer was used for the photographing (Tanon, Shanghai, China), the glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as the internal reference, the ratio of optical densities of the target protein and GAPDH was then calculated.
Total RNA extraction and real-time RT-PCR
The total RNA was extracted referring to the manual of TRI kit (Molecular Research Center, Cincinnati, USA). The RNA concentration was calculated by measuring the absorbance at 260 nm and 280 nm. 1 ug RNA was then used for the synthesis of corresponding cDNA with the reverse transcriptase Avian Myeloblastosis Virus (AMV; Promega Corporation, USA). The synthesis of syt-1 and SNAP-25-specific primers (KangCheng, Shanghai, China) was shown in Table 1, the main processes were as follows: pre-denaturation at 95°C for 5 min; denaturation at 95°C for 30 s, annealing at 60°C for 30 s, extension at 72°C for 30 s, with a total of 30 cycles. All reactions were carried out with the LightCycler® 480 instrument (Roche Diagnostics GmbH, Mannheim, Germany). The comparative CT-method was used to determine the amount of target, and expression levels were normalized to GAPDH.
Table 1.
Oligonucleotide sequence of primers for RT-PCR
| Genes | Sequences | Sizes (bp) |
|---|---|---|
| Syt-1 | Forward: 5’-CTTGTCCCACACAATGCCACT-3’ | 150 |
| Reverse: 5’-AAGGACCGCAACTATGGCT-3’ | ||
| SNAP-25 | Forward: 5’-GTTGGATGAGCAAGGCGAAC-3’ | 122 |
| Reverse: 5’-ACACACAAAGCCCGCAGAAT-3’ | ||
| GAPDH | Forward: 5’-AGTGCCAGCCTCGTCTCATA-3’ | 91 |
| Reverse: 5’-GAGAAGGCAGCCCTGGTAAC-3’ |
Results
Serum THs levels in rats
The serum radioimmunoassay test revealed that compared with the CON group, the mean T3 and T4 levels of the Hypo group and the DON group were significantly lower (P<0.0001); while those of the T4 group and the T4+DON group were close to the CON group (Table 2).
Table 2.
Comparison of serum T3 and T4 levels among the groups (X̅±s)
| Group | n | T3 (nmol/L) | T4 (nmol/L) |
|---|---|---|---|
| CON | 12 | 0.35±0.07 | 76.19±6.07 |
| Hypo | 12 | 0.19±0.05** | 21.75±2.50** |
| T4 | 12 | 0.36±0.05 | 75.03±4.95 |
| DON | 12 | 0.18±0.07** | 20.58±2.73** |
| T4+DON | 12 | 0.37±0.10 | 74.50±4.81 |
CON, Control group; Hypo, hypothyroid group; T4, hypothyroid rats treated with 5 µg T4/100 g body weight; DON, hypothyroid rats treated with 0.005% (w/v) DON in drinking water; T4+DON, hypothyroid rats treated with 5 µg T4/100 g BW and 0.005% (w/v) DON in drinking water. All data were presented as means ± S.E.M.
p<0.01 vs. the Control group.
TEM observation neuronal changes
The neurons of the CON group exhibited smooth and intact nuclear membranes, with chromatins evenly distributed; the mitochondria developed well, with clear internal ridge structures; the rough endoplasmic reticulum and ribosomes were rich (Figure 1A). The neurons of the Hypo group exhibited the margination of nuclear chromatins; a lot of mitochondria swelled, the cristae broke, the intimal area was reduced, and showed the significant vacuolar degeneration; the rough endoplasmic reticulum and ribosomes were obviously sparse (Figure 1B). The neurons of the T4 group and the DON group exhibited clear nuclear membranes, while the organelles were relatively sparse, and a small amount of mitochondria exhibited the vacuolation, partial rough endoplasmic reticulum was mildly dilated (Figure 1C, 1D). The morphologies of neurons, mitochondria, rough endoplasmic reticulum and ribosomes of the T4+DON groups were similar to the CON group. (Figure 1E).
Figure 1.
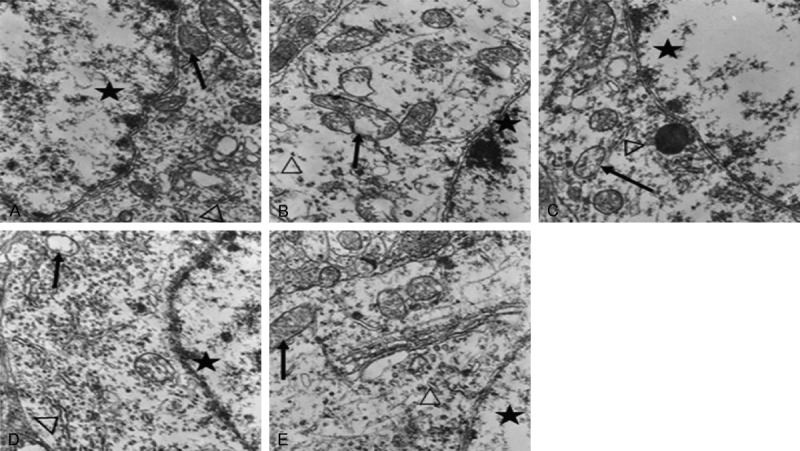
Ultrastructural observation of hippocampal neurons among the groups by TEM ×15 000. A: CON group; B: Hypo group; C: T4 group; D: DON group; E: T4+DON group; ★: neuronal nuclei; ↑: mitochondria; Δ: rough endoplasmic reticulum or ribosomes.
Synaptic changes
The structures of presynaptic membrane, synaptic cleft and postsynaptic membrane of the CON group were clear, the specialized belt was obvious, the synaptic vesicles were rich, and exhibited clear and dense-core vesicles (Figure 2A). While the presynaptic membrane and the postsynaptic membrane of the Hypo group were fused, the synaptic vesicles were significantly reduced, and almost no clear-type synaptic vesicles could be seen (Figure 2B). The presynaptic membrane and the postsynaptic membrane of the T4 group and the DON group were vague, the synaptic cleft was clear, while the number of clear-type synaptic vesicles was reduced (Figure 2C, 2D). The structures of three synaptic layers of the T4+DON group were relatively clear, the synaptic vesicles were relatively rich, close to the situations of the CON group (Figure 2E).
Figure 2.
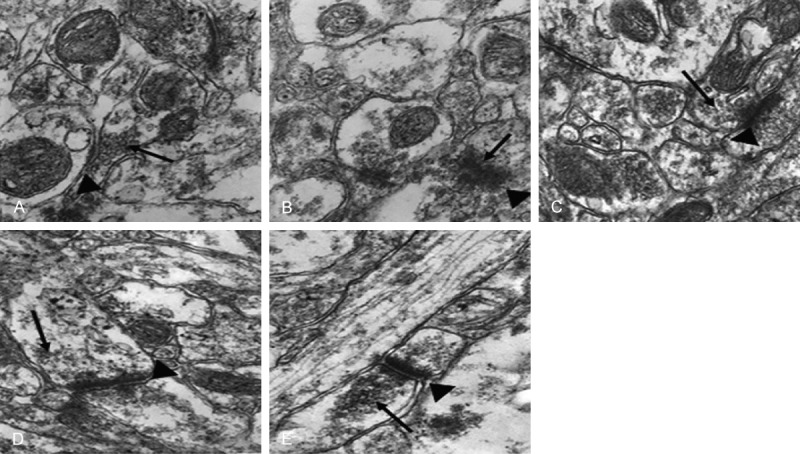
Observation of hippocampal synaptic ultrastructures of the groups by TEM ×40 000. A: CON group; B: Hypo group; C: T4 group; D: DON group; E: T4+DON group; (▲): synaptic structure; (↑): synaptic vesicles.
Western blot analysis
The results of Western blot analysis (Figure 3) revealed that the protein expressions of syt-1 and SNAP-25 were severely affected by the deficiency of THs. Compared with the CON group, the hippocampal syt-1 protein expression of the Hypo group was significantly reduced by 51.6% (P<0.0001), and those of the T4 group and the DON group were reduced by 19.9% and 30.0% (P=0.034 and 0.002), respectively, while that of the T4+DON group showed no difference with the CON group (P=0.901) (Figure 3A and 3C). Conversely, the expression of SNAP-25 protein of the Hypo group was significantly increased than the CON group by 56.8 % (P<0.0001), and only increased by 29.2% and 32.1% after the T4 and DON treatment (P=0.015, P=0.015), respectively, after the T4+DON treatment, the expression of SNAP-25 protein was restored to the normal level (P=0.493) (Figure 3B and 3D).
Figure 3.
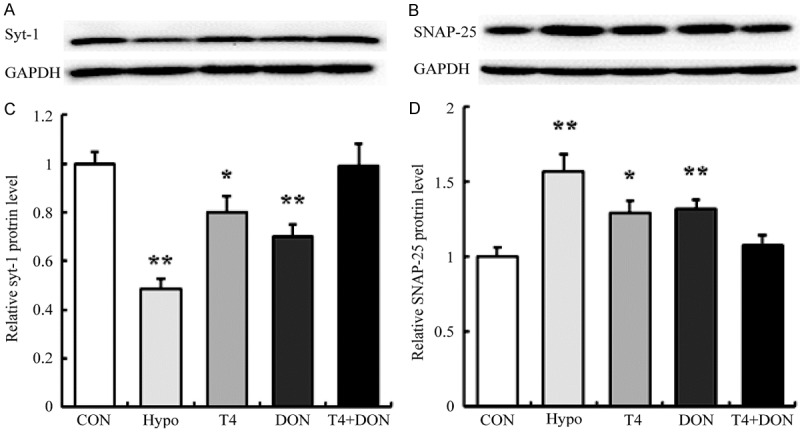
Thyroxine and donepezil regulated the expression of syt-1 and SNAP-25 protein in the dorsal hippocampus of adult hypothyroid rats. A, B. Western blot analysis towards the expressions of syt-1 and SNAP-25 protein in the dorsal hippocampus of CON, Hypo, T4, DON, and T4+DON groups (n=8). C, D. Quantification analysis towards the relative protein levels in each group. The data were shown as mean ± SEM. CON, Control group; Hypo, hypothyroid group; T4, hypothyroid rats treated with 5 µg T4/100 g BW; DON, hypothyroid rats treated with 0.005% (w/v) DON in drinking water; T4+DON, hypothyroid rats treated with 5 µg T4/100 g BW and 0.005% (w/v) DON in drinking water. *P<0.05, **P<0.01 vs. the Control group.
Real-time RT-PCR
Real-time RT-PCR (Figure 4) was used to detect the relative mRNA expression of syt-1 and SNAP-25 in the dorsal hippocampus. The results showed that the mRNA level of syt-1 was significantly decreased by 52.6% in the Hypo group (P=0.001), and decreased by 31.0% and 37.3% in the T4 group and the DON group (P=0.043 and 0.016), respectively, while no significant difference was found in the T4+DON group than the CON group (P=0.714) (Figure 4A). As for SNAP-25, the mRNA levels were found increased in both the Hypo group and the DON group by 37.5% and 31.6% (P=0.012 and 0.031), respectively, and the amount was observed to be restored to the normal value in the T4 group (P=0.081) and the T4+DON group (P=0.394) (Figure 4B).
Figure 4.
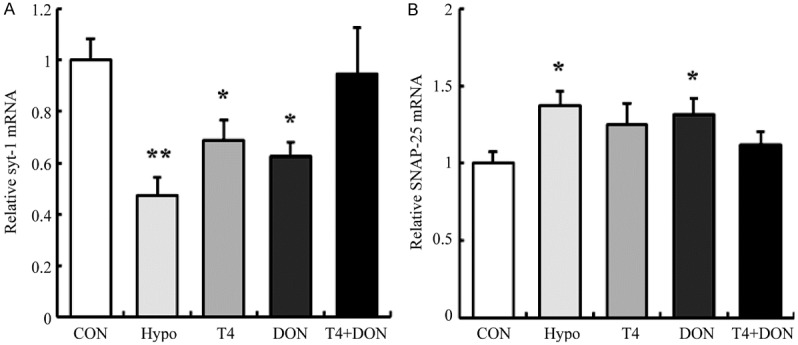
Thyroxine and donepezil regulated the mRNA expressions of syt-1 and SNAP-25 in the dorsal hippocampus of adult hypothyroid rats. Real-time RT-PCR analyzed the expressions of syt-1 (A) and SNAP-25 (B) mRNA in the dorsal hippocampus of CON, Hypo, T4, DON, and T4+DON groups (n=8). All data were presented as mean ± SEM. CON, Control group; Hypo, hypothyroid group; T4, hypothyroid rats treated with 5 µg T4/100 g BW; DON, hypothyroid rats treated with 0.005% (w/v) DON in drinking water; T4+DON, hypothyroid rats treated with 5 µg T4/100 g BW and 0.005% (w/v) DON in drinking water. *P<0.05, **P<0.01 vs. Control group.
Discussion
Hippocampus was vulnerable to be damaged by hypothyroidism in the adult stage [3], our findings contributed new evidence that adult hypothyroidism was harmful for this organ. The mitochondria inside neurons exhibited swelling and degeneration, the rough endoplasmic reticulum dilated, and the free ribosomes were obviously sparse. David found that the rough endoplasmic reticulum, Golgi complex and mitochondria in the neurons of hypothyroid rats were expanded, and the number of ribosomes was reduced [17]. There were also experiments that reported the frontal lobe, striatum and hippocampal nerve mitochondria of hypothyroid rats exhibited damages under TEM [18,19]. In addition, this study also observed that the synapses exhibited the specialized-belt fusion, the structures became unclear, and the synaptic vesicles were reduced. Certain TEM results showed that some adult hypothyroid rats exhibited the multi-systematic neuronal postsynaptic changes, degradation of dendric neurons and reduction of postsynaptic density in their brains [20,21]; and in the newborn rats, the deficiency of THs would damage both the numbers and qualities of cerebellar synaptic tissues [22]; certain animal experiment revealed that hypothyroidism delayed the growth of synaptic membranes and the contents in neonatal rats [23]. Mitochondria and ribosomes were considered as the active sites towards the synthesis of energy and proteins [17], the complete synaptic morphology was the structural basis towards the information transferring among the neurons, thus the destruction of the above intraneuronal organelles and synaptic structures might cause the dysfunctions of multiple aspects such as energy metabolism and protein synthesis. Biochemical studies had indicated that the amounts of RNA and protein per cell had been altered in the CNS in hypothyroidism [24,25]. In the present study, we explored the expression of syt-1 and SNAP-25 protein in this condition.
Syt-1 and SNAP-25 were the synapse-associated proteins, and distributed on the surface of synaptic vesicles and presynaptic membranes. In this study, the results of immunoblotting method showed that, compared with the CON group, the expression of syt-1 protein in the Hypo group was reduced, so was the mRNA level. Wang reported the same results with our research, he found that in the cerebellum of rats with iodine deficiency and hypothyroidism, the syt-1 protein was downregulated [26]. Meanwhile, the results of this research showed that the expressions of SNAP-25 protein and mRNA were significantly increased. One animal experiment found that in rats with thyroid resection, the pituitary SNAP-25 protein expression was increased [27]. It was also reported that the expressions of SNAP 25 in the dorsal hippocampus, ventral hippocampus and prefrontal cortex of hypothyroid mice with mild cognitive impairment were increased [28], consistent to our study. These results strongly suggested that THs could regulate the protein synthesis inside brain [29]. The expression differences of THs could change the expressions of thyroid hormone receptors, thus directly affecting the expressions of the THs-targeting proteins [30]. However, previous studies suggested the level of syt1 was increased in neonatal rat hippocampus [31]. Moreover, SNAP-25 mRNA and protein levels were downregulated in the developing hypothyroid rat brain [32]. These different findings on synaptic proteins might be due to the different response to inadequate THs at different ages. Although syt-1 and SNAP-25 were necessary during the neurotransmitter releasing, our results showed that hypothyroidism in adult stage had different impacts towards the expressions of syt-1 and SNAP-25. Our previous studies also supported this finding [8]. However, the mechanisms that these synaptic proteins occurred different changes in hypothyroid still remained unclear. Syt-1 would bind the SNARE core complex, then interacted directly with SNAP-25, and jointly promoted the release of neurotransmitters, and participated the regulation of synaptic plasticities [7]. In adult hypothyroidism, the reduced expression of syt-1 might be caused by its much more dependence on presence of THs, whereas SNAP-25 might be compensatorily increased to enhance the vesicle exocytosis. In addition, the increasing of SNAP-25 might also be related to the THs deficiency-caused calpain reduction in adulthood, which was the intracellular Ca+-dependent proteolytic enzyme, could cleave SNAP-25 while could not cleave syt-1 [33].
The T4 alternative therapy was the standard solution towards the hypothyroidism treatment. This experiment found that after given conventional doses of T4 for 2 weeks, the adult Hypo rats exhibited recovery towards the damaged hippocampal neuronal mitochondria, rough endoplasmic reticulum, ribosomes and synaptic ultrastructures, the exppressions of syt-1 and SNAP-25 were partially restored, but the protein level failed to return to normal. Although the thyroid hormone replacement seemed to reverse the hypothyroidism-induced impairment of late-phase long-term potentiation, CREB protein, MAPKp44/42 protein and some hippocampal functions [10], there were still some conflicting reports regarding the abilities of T4 administration to remedy all of the hippocampal impairments produced by hypothyroidism. Alzoubi found that even six-week T4 replacement therapy failed to return the decreased PKCγ expression back to normal [34]. Montero found that the short-term THs therapy could restore some hippocampal functions, such as the classical conditioning, but the learning and memory-related synaptic plasticities were not restored [35]. However, the exact mechanism underlying this remained elusive. Some study showed that the serum THs concentration was much higher than the concentration in the central nervous system [36]. The phenomenon that the brain injury was not completely healed after the T4 treatment might be related to the fact that when the serum THs levels returned normal, the intracerebullar hormone dose was still insufficient. Our previous study found that, when given large-dose T4 shock therapy to the adult hypothyroid rats, the hippocampal syt-1 and SNAP-25 proteins could restore to the normal levels, but hyperthyroidism could be induced at the same time [8].
As for the T4+DON treatment group, the morphological features in the hippocampal neuronal mitochondria, rough endoplasmic reticulum, ribosomes and synaptic structure were similar to the CON group. In fact, after the single DON treatment, the damages of hippocampal neurons and synaptic ultrastructures of the hypothyroid rats recovered to some extent, suggesting that DON could improve the hippocampal damages induced by hypothyroidism. As one cholinesterase inhibitor, DON was mainly used to treat mild to moderate cognitive impairment, and in recent years, it was confirmed the independent neuroprotective effects [37,38]. The experiment showed that DON coud improve the streptozotocin-induced mitochondrial dysfunction [39], and could also prevent the hippocampal mitochondrial damages in the transgenic model [15]; the long-term high-dose DON therapy could also increase the postsynaptic densities of dentate gyrus molecular layer in AD mice [40]; maintain the vertebral neuronal dendritic branches in aged rats, increase the total dendritic length and spine densities [41,42]. Kotani found that DON could increase the contact among neurons and synaptic connections [43]. In addition, this study also showed that after T4+DON treatment, the expression of syt-1 inside the synaptosomes returned to normal. Certain study showed that the expressions of core protein NR1 on the surface of presynaptic and postsynaptic glutamate receptors were reduced by DON [44]; DON was also reported to be able to induce the anti-inflammatory effects of acetylcholine, thus improving the hippocampal synaptic protein expressions in Tau rats [45]; our previous immunohistochemical study found that DON was beneficial towards the damages of such synaptic proteins as munc18 and syntaxin-1 induced by hypothyroidism [46]. The recovery of these synaptic proteins might also the reflect of DON’s nerve protective effects. There existed many mechanisms towards DON’s neuroprotective effects, some scholars believed that DON could confront the glutamate excitotoxicity by stimulating the α7 nicotinic receptors and internalize the NMDA receptors [44]; some scholars believed that DON played its neuroprotective effects by activating the hippocampal nutrient receptors [47]; another research reported that DON could improve the GABA energy input towards the hippocampal CA1 pyramidal neurons in aged rats, thus playing the neuroprotective effects [48].
In summary, adult hypothyroidism could cause the damages of hippocampal neurons and synaptic ultrastructures, as well as the expression level changes of syt-1 and SNAP-25 mRNA and protein inside the synapses, after DON or T4 treatment alone, the aforementioned damages were reversible, but the expressions of syt-1 and SNAP-25 failed to return to normal. After the T4+DON treatment, the expressions of syt-1 and SNAP-25 returned to normal, suggesting DON was valuable towards the hypothyroidism-induced hippocampal damages.
Acknowledgements
The department of toxicology of Anhui Medical University was greatly appreciated for the technical assistance. This study was supported by the National Natural Science Foundation of China (81272152).
Disclosure of conflict of interest
None.
References
- 1.Osterweil D, Syndulko K, Cohen SN, Pettler-Jennings PD, Hershman JM, Cummings JL, Tourtellotte WW, Solomon DH. Cognitive function in non-demented older adults with hypothyroidism. J Am Geriatr Soc. 1992;40:325–335. doi: 10.1111/j.1532-5415.1992.tb02130.x. [DOI] [PubMed] [Google Scholar]
- 2.Constantinou C, Margarity M, Valcana T. Region-specific effects of hypothyroidism on the relative expression of thyroid hormone receptors in adult rat brain. Mol Cell Biochem. 2005;278:93–100. doi: 10.1007/s11010-005-6934-z. [DOI] [PubMed] [Google Scholar]
- 3.Koromilas C, Liapi C, Schulpis KH, Kalafatakis K, Zarros A, Tsakiris S. Structural and functional alterations in the hippocampus due to hypothyroidism. Metab Brain Dis. 2010;25:339–354. doi: 10.1007/s11011-010-9208-8. [DOI] [PubMed] [Google Scholar]
- 4.Chen GH, Wang YJ, Qin S, Yang QG, Zhou JN, Liu RY. Age-related spatial cognitive impairment is correlated with increase of synaptotagmin 1 in dorsal hippocampus in SAMP8 mice. Neurobiol Aging. 2007;28:611–618. doi: 10.1016/j.neurobiolaging.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 5.Banerjee A, Kowalchyk JA, DasGupta BR, Martin TF. SNAP-25 is required for a late postdocking step in Ca2+-dependent exocytosis. J Biol Chem. 1996;271:20227–20230. doi: 10.1074/jbc.271.34.20227. [DOI] [PubMed] [Google Scholar]
- 6.Mehta PP, Battenberg E, Wilson MC. SNAP-25 and synaptotagmin involvement in the final Ca(2+)-dependent triggering of neurotransmitter exocytosis. Proc Natl Acad Sci U S A. 1996;93:10471–10476. doi: 10.1073/pnas.93.19.10471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chapman ER. Synaptotagmin: a Ca(2+) sensor that triggers exocytosis? Nature Rev Mol Cell Biol. 2002;3:498–508. doi: 10.1038/nrm855. [DOI] [PubMed] [Google Scholar]
- 8.Liu CL, Xu YX, Zhan Y, Hu HL, Jia XM, Chen GH, Zhu DF. Effect of thyroxine on synaptotagmin 1 and SNAP-25 expression in dorsal hippocampus of adult-onset hypothyroid rats. J Endocrinol Invest. 2011;34:280–286. doi: 10.1007/BF03347086. [DOI] [PubMed] [Google Scholar]
- 9.Miller KJ, Parsons TD, Whybrow PC, van Herle K, Rasgon N, van Herle A, Martinez D, Silverman DH, Bauer M. Memory improvement with treatment of hypothyroidism. Int J Neurosci. 2006;116:895–906. doi: 10.1080/00207450600550154. [DOI] [PubMed] [Google Scholar]
- 10.Alzoubi KH, Gerges NZ, Aleisa AM, Alkadhi KA. Levothyroxin restores hypothyroidism-induced impairment of hippocampus-dependent learning and memory: Behavioral, electrophysiological, and molecular studies. Hippocampus. 2009;19:66–78. doi: 10.1002/hipo.20476. [DOI] [PubMed] [Google Scholar]
- 11.Leentjens AF, Kappers EJ. Persistent cognitive defects after corrected hypothyroidism. Psychopathology. 1995;28:235–237. doi: 10.1159/000284933. [DOI] [PubMed] [Google Scholar]
- 12.Wekking EM, Appelhof BC, Fliers E, Schene AH, Huyser J, Tijssen JG, Wiersinga WM. Cognitive functioning and well-being in euthyroid patients on thyroxine replacement therapy for primary hypothyroidism. Eur J Endocrinol. 2005;153:747–753. doi: 10.1530/eje.1.02025. [DOI] [PubMed] [Google Scholar]
- 13.Samuels MH, Schuff KG, Carlson NE, Carello P, Janowsky JS. Health status, psychological symptoms, mood, and cognition in L-thyroxine-treated hypothyroid subjects. Thyroid. 2007;17:249–258. doi: 10.1089/thy.2006.0252. [DOI] [PubMed] [Google Scholar]
- 14.Cutuli D, De Bartolo P, Caporali P, Tartaglione AM, Oddi D, D’Amato FR, Nobili A, D’Amelio M, Petrosini L. Neuroprotective effects of donepezil against cholinergic depletion. Alzheimer’s Res Ther. 2013;5:50. doi: 10.1186/alzrt215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Riepe MW. Cholinergic treatment: what are the early neuropathological targets? Eur J Neurol. 2005;12(Suppl 3):3–9. doi: 10.1111/j.1468-1331.2005.01321.x. [DOI] [PubMed] [Google Scholar]
- 16.Zhao H, Qin HY, Cao LF, Chen YH, Tan ZX, Zhang C, Xu DX. Phenylbutyric acid inhibits epithelial-mesenchymal transition during bleomycin-induced lung fibrosis. Toxicol Lett. 2014;232:213–220. doi: 10.1016/j.toxlet.2014.10.013. [DOI] [PubMed] [Google Scholar]
- 17.David S, Nathaniel EJ. Neuronal changes induced by neonatal hypothyroidism: an ultrastructural study. Am J Anat. 1983;167:381–394. doi: 10.1002/aja.1001670308. [DOI] [PubMed] [Google Scholar]
- 18.Vega-Nunez E, Alvarez AM, Menendez-Hurtado A, Santos A, Perez-Castillo A. Neuronal mitochondrial morphology and transmembrane potential are severely altered by hypothyroidism during rat brain development. Endocrinology. 1997;138:3771–3778. doi: 10.1210/endo.138.9.5407. [DOI] [PubMed] [Google Scholar]
- 19.Stoica G, Lungu G, Xie X, Abbott LC, Stoica HM, Jaques JT. Inherited tertiary hypothyroidism in Sprague-Dawley rats. Brain Res. 2007;1148:205–216. doi: 10.1016/j.brainres.2007.02.042. [DOI] [PubMed] [Google Scholar]
- 20.Cortés C, Eugenin E, Aliaga E, Carreño LJ, Bueno SM, Gonzalez PA, Gayol S, Naranjo D, Noches V, Marassi MP, Rosenthal D, Jadue C, Ibarra P, Keitel C, Wohllk N, Court F, Kalergis AM, Riedel CA. Hypothyroidism in the adult rat causes incremental changes in brain-derived neurotrophic factor, neuronal and astrocyte apoptosis, gliosis, and deterioration of postsynaptic density. Thyroid. 2012;22:951–963. doi: 10.1089/thy.2010.0400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Madeira MD, Paula-Barbosa MM. Reorganization of mossy fiber synapses in male and female hypothyroid rats: a stereological study. J Comp Neurol. 1993;337:334–352. doi: 10.1002/cne.903370213. [DOI] [PubMed] [Google Scholar]
- 22.Hajos F, Patel AJ, Balazs R. Effect of thyroid deficiency on the synaptic organization of the rat cerebellar cortex. Brain Res. 1973;50:387–401. doi: 10.1016/0006-8993(73)90740-3. [DOI] [PubMed] [Google Scholar]
- 23.Lu EJ, Brown WJ. An electron microscopic study of the developing caudate nucleus in euthyroid and hypothyroid states. Anat Embryol. 1977;150:335–364. doi: 10.1007/BF00318351. [DOI] [PubMed] [Google Scholar]
- 24.Balazs R. Biochemical effects of thyroid hormones in the developing brain. UCLA Forum Med Sci. 1971;14:273–320. [PubMed] [Google Scholar]
- 25.Geel SE, Valcana T. Synthesis of free and membrane-bound ribosomal RNA from cerebral cortex in hypothyroid rats during development. Neurobiology. 1972;2:21–30. [PubMed] [Google Scholar]
- 26.Wang Y, Zhong J, Wei W, Gong J, Dong J, Yu F, Wang Y, Chen J. Developmental iodine deficiency and hypothyroidism impair neural development, upregulate caveolin-1, and downregulate synaptotagmin-1 in the rat cerebellum. Biol Trace Elem Res. 2011;144:1039–1049. doi: 10.1007/s12011-011-9089-7. [DOI] [PubMed] [Google Scholar]
- 27.Quintanar JL, Salinas E. Effect of hypothyroidism on synaptosomal-associated protein of 25 kDa and syntaxin-1 expression in adenohypophyses of rat. J Endocrinol Invest. 2002;25:754–758. doi: 10.1007/BF03345507. [DOI] [PubMed] [Google Scholar]
- 28.Cao L, Jiang W, Wang F, Yang QG, Wang C, Chen YP, Chen GH. The reduced serum free triiodothyronine and increased dorsal hippocampal SNAP-25 and Munc18-1 had existed in middle-aged CD-1 mice with mild spatial cognitive impairment. Brain Res. 2013;1540:9–20. doi: 10.1016/j.brainres.2013.09.034. [DOI] [PubMed] [Google Scholar]
- 29.Hayase K, Naganuma Y, Moriyama M, Yoshida A, Yokogoshi H. Effect of a thyroid hormone treatment on brain protein synthesis in rats. Biosci Biotechnol Biochem. 1997;61:1536–1540. doi: 10.1271/bbb.61.1536. [DOI] [PubMed] [Google Scholar]
- 30.Vallortigara J, Alfos S, Micheau J, Higueret P, Enderlin V. T3 administration in adult hypothyroid mice modulates expression of proteins involved in striatal synaptic plasticity and improves motor behavior. Neurobiol Dis. 2008;31:378–385. doi: 10.1016/j.nbd.2008.05.015. [DOI] [PubMed] [Google Scholar]
- 31.Vara H, Martinez B, Santos A, Colino A. Thyroid hormone regulates neurotransmitter release in neonatal rat hippocampus. Neuroscience. 2002;110:19–28. doi: 10.1016/s0306-4522(01)00541-3. [DOI] [PubMed] [Google Scholar]
- 32.Zhang HM, Su Q, Luo M. Thyroid hormone regulates the expression of SNAP-25 during rat brain development. Mol Cellular Biochem. 2008;307:169–175. doi: 10.1007/s11010-007-9596-1. [DOI] [PubMed] [Google Scholar]
- 33.Ando K, Kudo Y, Takahashi M. Negative regulation of neurotransmitter release by calpain: a possible involvement of specific SNAP-25 cleavage. J Neurochem. 2005;94:651–658. doi: 10.1111/j.1471-4159.2005.03160.x. [DOI] [PubMed] [Google Scholar]
- 34.Alzoubi KH, Gerges NZ, Alkadhi KA. Levothyroxin restores hypothyroidism-induced impairment of LTP of hippocampal CA1: electrophysiological and molecular studies. Exp Neurol. 2005;195:330–341. doi: 10.1016/j.expneurol.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 35.Fernandez-Lamo I, Montero-Pedrazuela A, Delgado-Garcia JM, Guadano-Ferraz A, Gruart A. Effects of thyroid hormone replacement on associative learning and hippocampal synaptic plasticity in adult hypothyroid rats. Eur J Neurosci. 2009;30:679–692. doi: 10.1111/j.1460-9568.2009.06862.x. [DOI] [PubMed] [Google Scholar]
- 36.van Doorn J, Roelfsema F, van der Heide D. Concentrations of thyroxine and 3,5,3’-triiodothyronine at 34 different sites in euthyroid rats as determined by an isotopic equilibrium technique. Endocrinology. 1985;117:1201–1208. doi: 10.1210/endo-117-3-1201. [DOI] [PubMed] [Google Scholar]
- 37.Chen K, Wang PP, Sun B, Li Q, Perruccio A, Power D, Wang C, He M, M H, Shibei Y, Krahn M, Cheung A, Hao X. Twenty-year secular changes in sex specific lung cancer incidence rates in an urban Chinese population. Lung Cancer. 2006;51:13–19. doi: 10.1016/j.lungcan.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 38.Akasofu S, Kimura M, Kosasa T, Sawada K, Ogura H. Study of neuroprotection of donepezil, a therapy for Alzheimer’s disease. Chem Biol Interact. 2008;175:222–226. doi: 10.1016/j.cbi.2008.04.045. [DOI] [PubMed] [Google Scholar]
- 39.Saxena G, Patro IK, Nath C. ICV STZ induced impairment in memory and neuronal mitochondrial function: A protective role of nicotinic receptor. Behav Brain Res. 2011;224:50–57. doi: 10.1016/j.bbr.2011.04.039. [DOI] [PubMed] [Google Scholar]
- 40.Dong H, Yuede CM, Coughlan CA, Murphy KM, Csernansky JG. Effects of donepezil on amyloid-beta and synapse density in the Tg2576 mouse model of Alzheimer’s disease. Brain Res. 2009;1303:169–178. doi: 10.1016/j.brainres.2009.09.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Alcántara-González F, Mendoza-Perez CR, Zaragoza N, Juarez I, Arroyo-García LE, Gamboa C, De La Cruz F, Zamudio S, Garcia-Dolores F, Flores G. Combined administration of cerebrolysin and donepezil induces plastic changes in prefrontal cortex in aged mice. Synapse. 2012;66:938–949. doi: 10.1002/syn.21588. [DOI] [PubMed] [Google Scholar]
- 42.Alcantara-Gonzalez F, Juarez I, Solis O, Martinez-Tellez I, Camacho-Abrego I, Masliah E, Mena R, Flores G. Enhanced dendritic spine number of neurons of the prefrontal cortex, hippocampus, and nucleus accumbens in old rats after chronic donepezil administration. Synapse. 2010;64:786–793. doi: 10.1002/syn.20787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kotani S, Yamauchi T, Teramoto T, Ogura H. Donepezil, an acetylcholinesterase inhibitor, enhances adult hippocampal neurogenesis. Chem Biol Interact. 2008;175:227–230. doi: 10.1016/j.cbi.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 44.Shen H, Kihara T, Hongo H, Wu X, Kem WR, Shimohama S, Akaike A, Niidome T, Sugimoto H. Neuroprotection by donepezil against glutamate excitotoxicity involves stimulation of alpha7 nicotinic receptors and internalization of NMDA receptors. Br J Pharmacol. 2010;161:127–139. doi: 10.1111/j.1476-5381.2010.00894.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yoshiyama Y, Kojima A, Ishikawa C, Arai K. Anti-inflammatory action of donepezil ameliorates tau pathology, synaptic loss, and neurodegeneration in a tauopathy mouse model. J Alzheimer’s Dis. 2010;22:295–306. doi: 10.3233/JAD-2010-100681. [DOI] [PubMed] [Google Scholar]
- 46.Wang N, Cai Y, Wang F, Zeng X, Jia X, Tao F, Zhu D. Effects of thyroxin and donepezil on hippocampal acetylcholine content and syntaxin-1 and munc-18 expression in adult rats with hypothyroidism. Exp Ther Med. 2014;7:529–536. doi: 10.3892/etm.2014.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Autio H, Mätlik K, Rantamäki T, Lindemann L, Hoener MC, Chao M, Arumäe U, Castrén E. Acetylcholinesterase inhibitors rapidly activate Trk neurotrophin receptors in the mouse hippocampus. Neuropharmacology. 2011;61:1291–1296. doi: 10.1016/j.neuropharm.2011.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Potier B, Jouvenceau A, Epelbaum J, Dutar P. Age-related alterations of GABAergic input to CA1 pyramidal neurons and its control by nicotinic acetylcholine receptors in rat hippocampus. Neuroscience. 2006;142:187–201. doi: 10.1016/j.neuroscience.2006.06.040. [DOI] [PubMed] [Google Scholar]


