Abstract
Context: As many studies proved that sodium iodide symporter (NIS) plays a key role in radioactive iodide (RAI) therapy of thyroid cancer, however, a growing number of studies suggests that part of differentiated thyroid carcinomas (DTC) with overexpression of NIS are insensitive to RAI well. Objective: The aim of this meta-analysis is to assess the expression of NIS in differentiated thyroid cancer, compared with normal thyroid tissue. Data Sources: PUBMED, Sinomed, CNKI, Wanfang and VIP were searched for relevant case-control studies up to now. Study Selection: Studies that concerning the qualitative expression NIS in DTC were included. Data Extraction: Working independently, authors used a standard form to extract data. For quality assessment, Newcastle-Ottawa Scale (NOS) were applied. Data Synthesis: Totally nine eligible studies included, involving 765 cases and 473 controls. The results revealed that the expression of NIS had a statistically increased in DTC, compared with controls (OddsRadio OR: 1.47, 95% CI: 1.12 to 1.94, Z=2.78, P=0.005). Since the existence of the significant heterogeneity, subgroup analysis and sensitivity analysis were performed and found that the heterogeneity came from the different criteria evaluate positive NIS expression (Liu 2008, Mu 2010) and the small simple size of the control group (Lin. J D2001). The heterogeneity disappeared or dropped to below 50% after remove these studies. Conclusion: Our study shows that the expression of NIS is significantly increased in DTC, which could help explain the reason for individual with a poor response to RAI therapy. In other word, the reduced iodide uptake in thyroid cancer may not caused by the decreased expression of NIS, function of NIS protein or its post-transcriptional translocation might be the point.
Keywords: Differentiated thyroid cancer, sodium iodide symporter, meta-analysis
Introduction
Thyroid cancer is a common endocrine malignancy that has rapidly increased in global incidence in recent decades. Relevant research showed that among all malignancies, thyroid cancer is now the fifth most frequent cancer for women in the USA. It is estimated that there were 60,220 new cases (14,910 men and 45,310 women) of thyroid cancer in 2013 and that a half million people are currently living with thyroid cancer in the USA [1]. Histopathologically, thyroid cancers can be classified into papillary, follicular, medullary, and anaplastic cancers [2]. Among them, papillary (PTC) and follicular carcinomas (FTC) are collectively known as differentiated thyroid cancer (DTC), which is derived from thyroid follicular epithelial cells and account for 97 percent of thyroid cancer. DTC is characterized by slow growth and a good prognosis, and improvements in diagnosis and treatment, due to the use of radioiodine, have reduced DTC-associated mortality [3]. Compared with medullary and anaplastic thyroid cancer, the prognosis of DTC is better.
Sodium-iodide symporter (NIS) is an integral protein of the basolateral membrane of thyroid gland follicular cells, which transports two Na+ for each I- into thyroid follicular cells [4]. So it is used to treat thyroid tumors by transport of radioiodine into cancer cells.
It is well known that the treatment of thyroid cancer including thyroidectomy, thyroid hormone inhibiting therapy and radioactive iodine (RAI) therapy. Of which, RAI could removes all remnant or residual normal thyroid tissues, is an important element of therapy following initial surgery in patients with papillary and follicular thyroid carcinomas [2]. The RAI treatment of thyroid cancer is based on the ability of thyroid follicular cells to concentrate iodine, which is dependent on the functional NIS [5]. Previous studies discovered that iodide uptake was depressed in DTC. But the expression of NIS was disputable in DTC. Quite numbers of studies have demonstrated that the low expression of NIS protein in DTC, which may be responsible for the decreased iodide accumulation in thyroid carcinomas [6-8]. However, many others discovered a normal or even overexpression in DTC [5,9-11].
The aim of the present meta-analysis was to assess the expression of NIS in DTC, in order to point a direction for improving the efficacy in those refractory to iodine radiation therapy.
Materials and methods
Literature search
A comprehensive literature search was performed using the PubMed, Sinomed CNKI, VIP and Wanfang database for relevant articles published up to now with the following search terms: (thyroid neoplasm OR thyroid cancer OR thyroid carcinoma OR thyroid cancer, papillary OR thyroid cancer, medullary OR thyroid cancer, follicular OR thyroid cancer, anaplastic) AND (sodium iodide symporter OR NIS OR thyroid iodide symporter OR SLC5A5). Additional studies were identified by hand searching references in original articles and review articles.
Study selection
We included any study that met all of the following criteria: 1) published in English or Chinese language; 2) the study design was a case-control study; 3) investigated the association between qualitative expression of sodium iodide symporter and thyroid cancer; 4) the diagnosis of thyroid cancer was confirmed either histological, pathologically or cytological; the positive expression of NIS is detected by immunohistochemistry technology; 5) the odds ratios (OR) and the corresponding 95% confidence intervals (CIs), or the number of positive events that can calculate them were reported. Studies not designed as case control studies (randomized control studies, systematic review, case report and so on), no or incomplete data provided, animal tests were excluded from this meta-analysis. The eligibility of included studies was evaluated by two investigators independently based on the predetermined selection criteria.
Data extraction
Two reviewers independently extracted the following data for each eligible study: first author’s last name, study period, year of publication, characteristics of observed subjects (including age, sex radio), site of origin, histological type of the tumor, source of cases and controls, number of cases and controls, the number of samples that show positive expression of NIS and the total quantity. Any disagreements were resolved by consensus.
Study quality assessment
The quality of the studies was also independently assessed by the same two reviewers according to Newcastle-Ottawa Scale (NOS) [12], a validated quality assessment instrument for nonrandomized trials, which consists of three parameters of quality: selection, comparability, and exposure assessment. The NOS assigns a maximum score of 4 for selection, 2 for comparability, and 3 for exposure. Studies with a NOS score of five or greater were regarded as moderate to high quality studies, the best score is 9, whereas those with a NOS score of less than five score were considered low quality studies.
Statistical analyses and publication bias
Statistical analyses were performed using Review Manager 5.2 (Cochrane Collaboration, United Kingdom). A P-value <0.05 was considered statistical significance for odds risk (OR). The combined OR and 95% confidence interval (CI) were used for analysis. Meanwhile, forest plots were generated to determine whether there was a statistical association between cases and controls and to assess heterogeneity of the included studies. Heterogeneity was quantified evaluated using the I2 statistic, this statistic yields results ranged from 0 to 100% (I2=0-25%, no heterogeneity; I2=25-50%, moderate heterogeneity; I2=50-75%, large heterogeneity; and I2=75-100%, extreme heterogeneity) [13]. If heterogeneity existed, the random effects model was used, otherwise, the fixed effects model was used. If significant heterogeneity is identified, subgroup analysis and sensitivity analysis were also performed according to the following characteristic: research areas; histological type of the tumor, source of controls, and continent in which the study was conducted (America and Asia). Visual inspection of asymmetry in funnel plot was performed. Egger’s regression method was also used for statistical assessment of publication bias (P<0.05 was considered representative of statistically significant publication bias) [14].
Results
Identification of eligible studies
The initial database search identified 1240 papers. Of these, 37 papers describing the expression of NIS in thyroid cancer were identified for further evaluation after scanning the titles and abstracts. Finally, nine studies [15-23] were included based on the inclusive criteria after full-text review (Searching progress was summarized in Figure 1).
Figure 1.
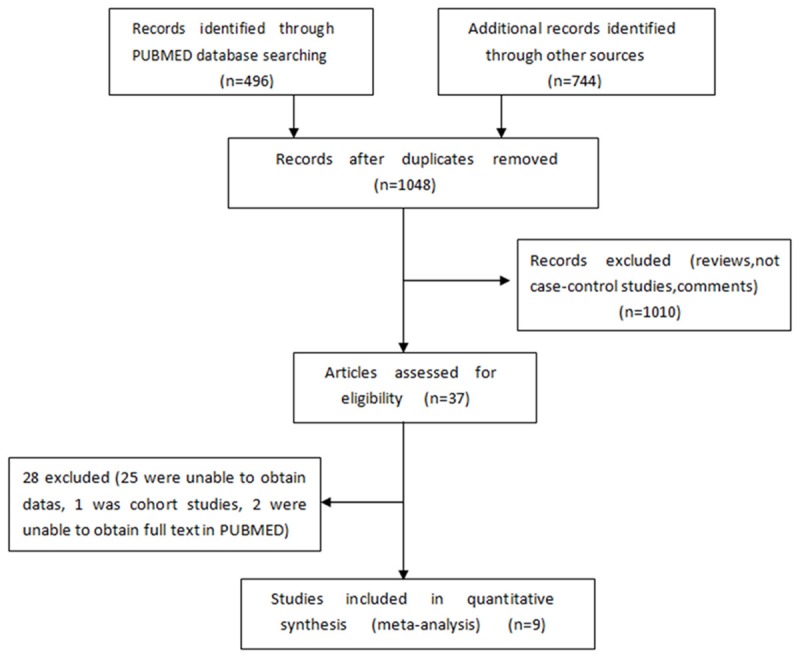
Summary of the Studies Selection Process Characteristics of included studies.
The main characteristics and results of the nine papers were summarized in Table 1. Among the nine studies, eight were conducted in China, one in Brazil. The sample size ranged from 17 to 265 in thyroid cancer group while 3 to 135 in control group. All of the cases were histological, pathologically or cytological confirmed as differentiated thyroid cancer, of them, one study did not distinguish the type of DTC, the other eight studies clearly indicated the type (PTC or FTC). Of all nine studies, the NOS assigns a maximum score of 4 for selection, 2 for comparability, and 3 for exposure. Studies with a NOS score of five or greater were regarded as moderate to high quality studies, the best score is nine, whereas those with a NOS score of less than five score were considered low quality studies. The NOS score for each study was also listed in Table 1.
Table 1.
Characteristics, Statistical Data and NOS Score of Included Studies
| Author (Year) | Study period | Area | Age range | Gender | NO. of case (PTC/FTC) | NO.of control (NTT) | NIS (+) | NOS score |
|---|---|---|---|---|---|---|---|---|
| Lin, J.D | ~2001 | TaiWan | 21-76y | Case M: 1, F: 16 | 17 | 3 (Ca) | Case: 4 | 5 |
| Liu Chun-Ping | 2003-2007 | WuHan | 21-72y | Case M: 13, F: 53 | 45 | 45 (NG, FA) | Case: 39 | 5 |
| (2008) | Control: not mentioned | Control: 45 | ||||||
| Mu za pa er | 2005-2009 | XinJiang | 9-76y | Case=control | 72 | 72 (Ca) | Case: 22 | 5 |
| (2010) | M: 16, F: 56 | Control: 58 | ||||||
| Hu Yang-Ying | 2006-2009 | NanJing | 13-71y | Case M: 14, F: 35 | 49 | 49 (Ca) | Case: 33 | 6 |
| (2010) | Control: not mentioned | Control: 28 | ||||||
| Morari, E.C | ~2011 | SaoPaulo | Not mentioned | Not mentioned | 265 | 18 (NG, FA) | Case: 32 | 6 |
| (2011) | Control: 1 | |||||||
| Wang Zhi-Feng | 2009-2010 | GanSu | 14-78y | Case M: 14, F: 35 | 56 | 52 (NG, FA) | Case: 51 | 6 |
| (2012) | Not mentioned | Control: 29 |
M: male; F: female; PTC: papillary thyroid cancer; FTC: follicular thyroid cancer; DTC: differentiated thyroid cancer; NTT: normal thyroid tissue; NG: nodular goiter; FA: follicular adenoma; NTT (Ca/NG, FA):normal thyroid tissue from paracarcinoma or nodular goiter, follicular adenoma.
NIS expression in differentiated thyroid cancer
Figure 2 showed the estimated pooled OR associated with expression of NIS in DTC. Significant heterogeneity was detected (I2=90%, P=0.005). The OR and 95%CI of each study can be seen in Figure 2. The pooled OR from all nine studies was 1.47 (95% CI: 1.12-1.94, P=0.005). Because of the significant heterogeneity, subgroup analysis and sensitivity analysis were conducted according to areas, source of case group, and source of control group and continents of the studies. The analysis showed that the heterogeneity came from the different criteria of evaluating positive NIS expression (Liu chun-ping 2008, Mu za pa er 2010) and the small simple size of the control group (Lin. J D2001). The heterogeneity disappeared or dropped to below 50% after remove these studies (Figure 3). The fixed-effect model was used to merge OR values. The pooled OR was 6.67 (95% CI: 3.47-12.80, I2=0%, Z=5.7, P<0.00001) for the control group which was composed of normal thyroid tissues from lobes of nodular goiter or follicular adenoma and not affected by cancer; The OR value of control group that normal thyroid tissue from paracarcinoma was 2.34 (95% CI: 1.54-3.58, I2=31%, Z=3.94, P<0.0001). Pooled data from all six studies showed that the NIS expression of DTC is larger than that of normal thyroid tissue. Funnel plot and Egger’s regression method (P=0.86, P>0.05 was considered representative of no statistically significant publication bias) showed that there was no significant publication bias exist (Figure 4).
Figure 2.
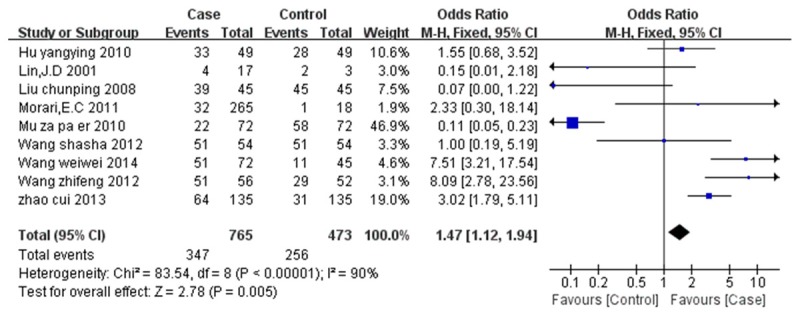
Forest Plot of Odds Radios and 95% CI of NIS expression in thyroid cancer compared with normal thyroid tissue.
Figure 3.
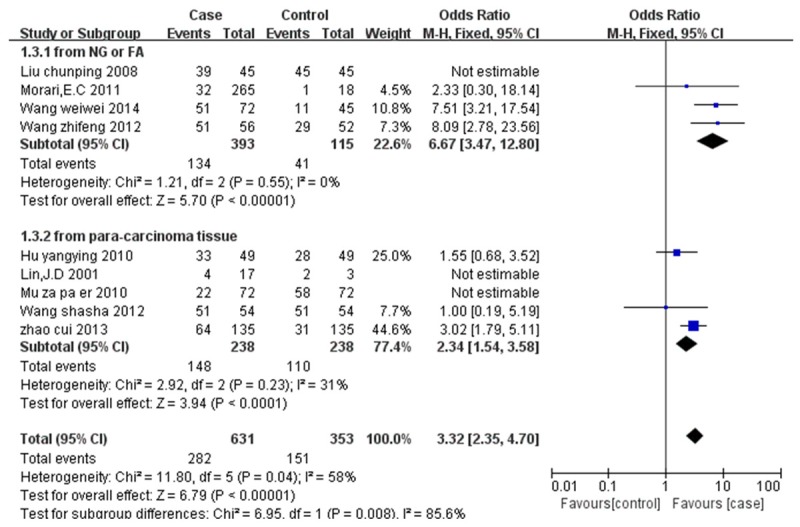
The expression of NIS in thyroid cancer compared with normal thyroid tissue: sensitivity analysis of OR.
Figure 4.
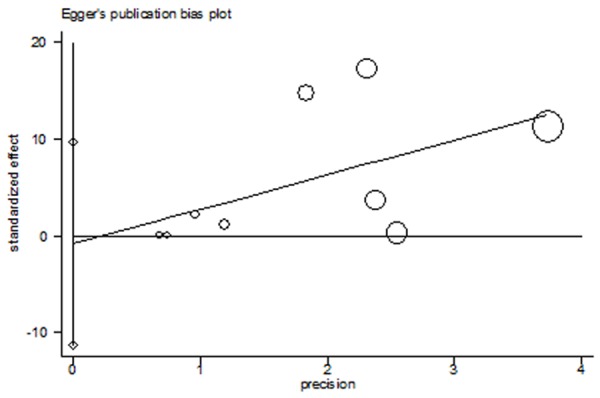
Egger’s test for NIS Expression in thyroid cancers compared with normal thyroid tissues.
Discussion
Dependent on the Na+ gradient maintained by Na+/K+ ATPase [24], NIS mediates the active transport of iodide in the thyroid gland and a number of non-thyroidal tissues, including mammary gland during lactation, stomach, salivary and lacrimal glands [25]. Particularly, the thyroid gland was found to be capable of concentrating iodide by a factor of 20-40 with respect to its plasma level [26].
NIS and NIS-mediated iodide accumulation in the thyroid gland represent crucial prerequisites for diagnostic thyroid scintigraphy, as well as therapeutic application for radioiodine in benign and malignant thyroid diseases [25]. Compared with anaplastic thyroid cancer, well differentiated thyroid cancer is common and most of them are sensitive to radioactive iodide therapy. However, studies shows that over 20%-30% of differentiated thyroid carcinomas insensitive to 131I treatment, resulting in the high recurrence rate and poor prognosis of these patients [11,27,28]. As mentioned above, the reasons for the decreased uptake of 131I in DTC is controversial. Some studies found that the expression of NIS is reduced or loss [29], while others found it sufficient or even overexpression [9,11,30,31].
Our study based on nine case-control studies shows that the protein expression of NIS protein in differentiated cancers is higher in DTC than that in normal thyroid tissues (P=0.005, I2=90%). Subgroup analysis and sensitivity analysis were conducted according to areas, source of case group, sources of control group and continents of the studies. The analysis showed that the heterogeneity came from the different criteria evaluating positive NIS expression (Liu chun-ping 2008, Mu za pa er 2010) and the small simple size of the control group (Lin. J D2001). The significant heterogeneity was missing or dropping to below 50% after remove these studies (Figure 3). Given the result of this analysis is consist with several previous studies [9,11,15,16,19-21,30-32], we may predict that the reduced uptake of 131I in a majority of differentiated thyroid cancers is mainly due to the defective localization of NIS protein.
Recently, a study shows that NIS protein levels in cancerous tissue was higher than that in normal thyroid tissue, while NIS mRNA levels in cancerous tissue was lower than in normal thyroid tissue. This suggested that abnormal expression of NIS at translational level is one of the main reasons for its high expression in tumor cells [15]. The localization of NIS to the basolateral membrane has been closely related to its functional role and associated with iodide-accumulating ability of follicular cells [36]. Previous studies that used immumnohistochemical staining indicated that most of NIS protein was expressed in the cytoplasm of neoplastic cells, not in its right place, the cytoplasmic membrane [9,29,34-38]. Posttranslational regulatory mechanisms, especially translation of NIS, have been considered as an important factor determining the function of NIS, and of interest as a target to augment iodide uptake in NIS-expressing cancer cells [39].
The translocation of NIS in thyroid cells is a complex process and regulated by multi-factors: thyroid-stimulating hormone (TSH) [40], TSH receptor (TSHR), PTTG1-binding factor (PBF) [41] and PI3K [42,43] have been investigated in the past few years. All of them are known to play an important role in the progress of NIS translocation to the cell membrane. Among of them, TSH and TSHR are two crucial factors in stimulating the NIS translocation to the cell surface membrane. TSH regulates every step of thyroid iodine metabolism through the cAMP cascade. Iodine uptake has been demonstrated to be under the control of TSH, as stimulation by TSH increases radioiodine uptake in vivo and in vitro as well as human NIS (hNIS) expression in cultured thyroid cell [41,44]. In the presence of TSH, NIS in FRTL-5 cells is mainly distributed to the cell surface membrane, while when TSH is excluded, NIS is mainly localized in the intracellular compartments [45]. Research also shows that TSHR plays a role in NIS translocation in thyroid tissues. In papillary thyroid cancer, high concentration of TSH combined with TSHR could improve the expression of NIS or mediate the NIS properly located in cell membrane, thus increasing the 131I uptake in therapy. PBF, one of the NIS upstream enhancer (NUE) regulators, it’s effect on the NIS expression was observed in Smith’s study [41] that exogenous PBF expression significantly reduced iodide uptake and cell surface NIS expression in NIS-introduced Cos-7 cells. In addition, PI3K was reported that a constitutively active mutant of PI3K, p110αCAAX, suppressed the expression of NIS in cell surface, as well as iodide uptake in MCF7 cells [44]. The underlying mechanism of how the above factors regulate the expression of NIS and the uptake of iodide is not clear.
Our study indicated that the expression of NIS is not the reason of low iodide uptake in Differentiated Thyroid Cancer. It might be caused by abnormal translation of NIS rather than reduced expression of NIS in thyroid carcinoma cells may play a key role in this process. Although our analysis shows that the expression of NIS in differentiated thyroid cancer is more than that in normal thyroid tissues, moreover, the publication bias of our included studies is not obvious (Figure 4), but several limitations should be considered: 1) the number of relevant studies included is restricted, so the sample size in case and control group is not large; 2) As indicated by I2 statistics, the studies included in our meta-analysis showed significant heterogeneity, once the studies that inducing discrepancy were removed for sensitivity analysis, the heterogeneity were deleted or reduced to an acceptable range.
In conclusion, our study suggests that the expression of NIS is significantly increased, and the expression of NIS is not the reason of low iodine uptake in DTC. More clinical or experimental researches with high-quality, larger sample and strictly case-control studies are needed to confirm our conclusion in the future. The underlying mechanisms that link NIS expression with effective RAI therapy also should be further investigated.
Acknowledgements
Natural Science Fund of Shandong Province (grant numbers: Y2006C76, Y2008C73, ZR2010HM044); Science and Technology Development Projects of Shandong Province (grant numbers: 2010GSF10228, 2012GGH11862); National Science Foundation (grant numbers: 81070637).
Disclosure of conflict of interest
None.
References
- 1.Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, Mandel SJ, Mazzaferri EL, McIver B, Pacini F, Schlumberger M, Sherman SI, Steward DL, Tuttle RM. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19:1167–1214. doi: 10.1089/thy.2009.0110. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 3.Fernandes JK, Day TA, Richardson MS, Sharma AK. Overview of the management of differentiated thyroid cancer. Curr Treat Options Oncol. 2005;6:47–57. doi: 10.1007/s11864-005-0012-3. [DOI] [PubMed] [Google Scholar]
- 4.Eskandari S, Loo DD, Dai G, Levy O, Wright EM, Carrasco N. Thyroid Na+/I- symporter. Mechanism, stoichiometry, and specificity. J Biol Chem. 1997;272:27230–27238. doi: 10.1074/jbc.272.43.27230. [DOI] [PubMed] [Google Scholar]
- 5.Arturi F, Russo D, Schlumberger M, du Villard JA, Caillou B, Vigneri P, Wicker R, Chiefari E, Suarez HG, Filetti S. Iodide symporter gene expression in human thyroid tumors. J Clin Endocrinol Metab. 1998;83:2493–2496. doi: 10.1210/jcem.83.7.4974. [DOI] [PubMed] [Google Scholar]
- 6.Trouttet-Masson S, Selmi-Ruby S, Bernier-Valentin F, Porra V, Berger-Dutrieux N, Decaussin M, Peix JL, Perrin A, Bournaud C, Orgiazzi J, Borson-Chazot F, Franc B, Rousset B. Evidence for transcriptional and posttranscriptional alterations of the sodium/iodide symporter expression in hypofunctioning benign and malignant thyroid tumors. Am J Pathol. 2004;165:25–34. doi: 10.1016/S0002-9440(10)63272-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gerard AC, Daumerie C, Mestdagh C, Gohy S, De Burbure C, Costagliola S, Miot F, Nollevaux MC, Denef JF, Rahier J, Franc B, De Vijlder JJ, Colin IM, Many MC. Correlation between the loss of thyroglobulin iodination and the expression of thyroid-specific proteins involved in iodine metabolism in thyroid carcinomas. J Clin Endocrinol Metab. 2003;88:4977–4983. doi: 10.1210/jc.2003-030586. [DOI] [PubMed] [Google Scholar]
- 8.Faggiano A, Caillou B, Lacroix L, Talbot M, Filetti S, Bidart JM, Schlumberger M. Functional characterization of human thyroid tissue with immunohistochemistry. Thyroid. 2007;17:203–211. doi: 10.1089/thy.2006.0174. [DOI] [PubMed] [Google Scholar]
- 9.Saito T, Endo T, Kawaguchi A, Ikeda M, Katoh R, Kawaoi A, Muramatsu A, Onaya T. Increased expression of the sodium/iodide symporter in papillary thyroid carcinomas. J Clin Invest. 1998;101:1296–1300. doi: 10.1172/JCI1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Luciani P, Buci L, Conforti B, Tonacchera M, Agretti P, Elisei R, Vivaldi A, Cioppi F, Biliotti G, Manca G, Vitti P, Serio M, Peri A. Expression of cAMP response element-binding protein and sodium iodide symporter in benign non-functioning and malignant thyroid tumours. Eur J Endocrinol. 2003;148:579–586. doi: 10.1530/eje.0.1480579. [DOI] [PubMed] [Google Scholar]
- 11.Dohan O, Baloch Z, Banrevi Z, Livolsi V, Carrasco N. Rapid communication: predominant intracellular overexpression of the Na(+)/I(-) symporter (NIS) in a large sampling of thyroid cancer cases. J Clin Endocrinol Metab. 2001;86:2697–2700. doi: 10.1210/jcem.86.6.7746. [DOI] [PubMed] [Google Scholar]
- 12.Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ottawa (ON): Ottawa Hospital Research Institute; 2009. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed 2012 Jan.) [Google Scholar]
- 13.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao C, et al. Association of expression of sodium iodide symporter and aggressive variants of papilliary thyroid carcinomas. http://d.wanfangdata.com.cn/Thesis Y2396963.aspx. [DOI] [PubMed]
- 16.Wang WW, Wang F, Fang SB, Ning CP, Yan SL, et al. The correlation between the expression of sodium iodide symporter and the characteristics of ultrasonography in differentiated thyroid carcinoma. Chin J Clinicians. 2014;8:1229–1233. [Google Scholar]
- 17.Liu CP, Chu HM, Ming J, Pan HX, Huang T, et al. Expression and clicinal significance of thyroid stimulating hormone receptor and Na+/I- symporter in human thyroid carcinoma. ACTAANATOMICA SINICA. 2008;39:756–759. [Google Scholar]
- 18.Mu Za Pa Er, Ma BL, et al. The expression and clinical significance of NIS in Xinjiang Uygur papillary adenocarcinoma. 2010. http://d.wanfangdata.com.cn/Thesis D175100.aspx.
- 19.Wang ZF, Liu QJ, Liao SQ, Yang R, Ge T, He X, Tian CP, Liu W. Expression and correlation of sodium/iodide symporter and thyroid stimulating hormone receptor in human thyroid carcinoma. Tumori. 2011;97:540–6. doi: 10.1177/030089161109700420. [DOI] [PubMed] [Google Scholar]
- 20.Wang SS, et al. Papillary thyroid carcinoma related genes expression and prognosis influenced by surgical procedure. 2012. http://d.wanfangdata.com.cn/Thesis Y2088297. aspx.
- 21.Hu YY, Shen MP, et al. The expression and location of sodium iodide symporter in differentiated thyroid carcinoma and corresponding lymph nodes metastasis. Acta Univ Med Nanjing. 2010;30:1082–1085. [Google Scholar]
- 22.Lin JD, Hsueh C, Chao TC, Weng HF. Expression of sodium iodide symporter in benign and malignant human thyroid tissues. Endocr Pathol. 2001;12:15–21. doi: 10.1385/ep:12:1:15. [DOI] [PubMed] [Google Scholar]
- 23.Morari EC, Marcello MA, Guilhen AC, Cunha LL, Latuff P, Soares FA, Vassallo J, Ward LS. Use of sodium iodide symporter expression in differentiated thyroid carcinomas. Clin Endocrinol (Oxf) 2011;75:247–54. doi: 10.1111/j.1365-2265.2011.04032.x. [DOI] [PubMed] [Google Scholar]
- 24.Dohan O, De la Vieja A, Paroder V, Riedel C, Artani M, Reed M, Ginter CS, Carrasco N. The sodium/iodide Symporter (NIS): characterization, regulation, and medical significance. Endocr Rev. 2003;24:48–77. doi: 10.1210/er.2001-0029. [DOI] [PubMed] [Google Scholar]
- 25.Spitzweg C, Morris JC. The sodium iodide symporter: its pathophysiological and therapeutic implications. Clin Endocrinol (Oxf) 2002;57:559–74. doi: 10.1046/j.1365-2265.2002.01640.x. [DOI] [PubMed] [Google Scholar]
- 26.Riggs DS. Quantitative aspects of iodine metabolism in man. Pharmacol Rev. 1952;4:284–370. [PubMed] [Google Scholar]
- 27.Antonelli A, Fallahi P, Ferrari SM, Carpi A, Berti P, Materazzi G, Minuto M, Guastalli M, Miccoli P. Dedifferentiated thyroid cancer: a therapeutic challenge. Biomed Pharmacother. 2008;62:559–63. doi: 10.1016/j.biopha.2008.07.056. [DOI] [PubMed] [Google Scholar]
- 28.Ward LS, Santarosa PL, Granja F, da Assumpcao LV, Savoldi M, Goldman GH. Low expression of sodium iodide symporter identifies aggressive thyroid tumors. Cancer Lett. 2003;200:85–91. doi: 10.1016/s0304-3835(03)00392-6. [DOI] [PubMed] [Google Scholar]
- 29.Min JJ, Chung JK, Lee YJ, Jeong JM, Lee DS, Jang JJ, Lee MC, Cho BY. Relationship between expression of the sodium/iodide symporter and 131I uptake in recurrent lesions of differentiated thyroid carcinoma. Eur J Nucl Med. 2001;28:639–45. [PubMed] [Google Scholar]
- 30.Wapnir IL, van de Rijn M, Nowels K, Amenta PS, Walton K, Montgomery K, Greco RS, Dohán O, Carrasco N. Immunohistochemical profile of the sodium/iodide symporter in thyroid, breast, and other carcinomas using high density tissue microarrays and conventional sections. J Clin Endocrinol Metab. 2003;88:1880–8. doi: 10.1210/jc.2002-021544. [DOI] [PubMed] [Google Scholar]
- 31.Wu XC, Chen VW, Steele B, Roffers S, Klotz JB, Correa CN, Carozza SE. Cancer incidence in adolescents and young adults in the United States, 1992-1997. J Adolesc Health. 2003;32:405–15. doi: 10.1016/s1054-139x(03)00057-0. [DOI] [PubMed] [Google Scholar]
- 32.Dong JJ, Zhou Y, Liu YT, Zhang ZW, Zhou XJ, Wang HJ, Liao L. In vitro evaluation of the therapeutic potential of nevirapine in treatment of human thyroid anaplastic carcinoma. Mol Cell Endocrinol. 2013;370:113–8. doi: 10.1016/j.mce.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 33.Dai G, Levy O, Carrasco N. Cloning and characterization of the thyroid iodide transporter. Nature. 1996;379:458–60. doi: 10.1038/379458a0. [DOI] [PubMed] [Google Scholar]
- 34.Arturi F, Russo D, Giuffrida D, Schlumberger M, Filetti S. Sodium-iodide symporter (NIS) gene expression in lymph-node metastases of papillary thyroid carcinomas. Eur J Endocrinol. 2000;143:623–7. doi: 10.1530/eje.0.1430623. [DOI] [PubMed] [Google Scholar]
- 35.Castro MR, Bergert ER, Goellner JR, Hay ID, Morris JC. Immunohistochemical analysis of sodium iodide symporter expression in metastatic differentiated thyroid cancer: correlation with radioiodine uptake. J Clin Endocrinol Metab. 2001;86:5627–32. doi: 10.1210/jcem.86.11.8048. [DOI] [PubMed] [Google Scholar]
- 36.Jung YH, Hah JH, Sung MW, Kim KH, Cho SY, Jeon YK. Reciprocal immunohistochemical expression of sodium/iodide symporter and hexokinase I in primary thyroid tumors with synchronous cervical metastasis. Laryngoscope. 2009;119:541–8. doi: 10.1002/lary.20073. [DOI] [PubMed] [Google Scholar]
- 37.Patel A, Jhiang S, Dogra S, Terrell R, Powers PA, Fenton C, Dinauer CA, Tuttle RM, Francis GL. Differentiated thyroid carcinoma that express sodium-iodide symporter have a lower risk of recurrence for children and adolescents. Pediatr Res. 2002;52:737–44. doi: 10.1203/00006450-200211000-00021. [DOI] [PubMed] [Google Scholar]
- 38.Sodre AK, Rubio IG, Galrao AL, Knobel M, Tomimori EK, Alves VA, Kanamura CT, Buchpiguel CA, Watanabe T, Friguglietti CU, Kulcsar MA, Medeiros-Neto G, Camargo RY. Association of low sodium-iodide symporter messenger ribonucleic acid expression in malignant thyroid nodules with increased intracellular protein staining. J Clin Endocrinol Metab. 2008;93:4141–5. doi: 10.1210/jc.2007-0353. [DOI] [PubMed] [Google Scholar]
- 39.Kogai T, Brent GA. The sodium iodide symporter (NIS): regulation and approaches to targeting for cancer therapeutics. Pharmacol Ther. 2012;135:355–70. doi: 10.1016/j.pharmthera.2012.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kogai T, Endo T, Saito T, Miyazaki A, Kawaguchi A, Onaya T. Regulation by thyroid-stimulating hormone of sodium/iodide symporter gene expression and protein levels in FRTL-5 cells. Endocrinology. 1997;138:2227–32. doi: 10.1210/endo.138.6.5189. [DOI] [PubMed] [Google Scholar]
- 41.Smith VE, Read ML, Turnell AS, Watkins RJ, Watkinson JC, Lewy GD, Fong JC, James SR, Eggo MC, Boelaert K, Franklyn JA, McCabe CJ. A novel mechanism of sodium iodide symporter repression in differentiated thyroid cancer. J Cell Sci. 2009;122:3393–402. doi: 10.1242/jcs.045427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kogai T, Sajid-Crockett S, Newmarch LS, Liu YY, Brent GA. Phosphoinositide-3-kinase inhibition induces sodium/iodide symporter expression in rat thyroid cells and human papillary thyroid cancer cells. J Endocrinol. 2008;199:243–52. doi: 10.1677/JOE-08-0333. [DOI] [PubMed] [Google Scholar]
- 43.Knostman KA, McCubrey JA, Morrison CD, Zhang Z, Capen CC, Jhiang SM. PI3K activation is associated with intracellular sodium/iodide symporter protein expression in breast cancer. BMC Cancer. 2007;7:137. doi: 10.1186/1471-2407-7-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Saito T, Endo T, Kawaguchi A, Ikeda M, Nakazato M, Kogai T, Onaya T. Increased expression of the Na+/I- symporter in cultured human thyroid cells exposed to thyrotropin and in Graves’ thyroid tissue. J Clin Endocrinol Metab. 1997;82:3331–6. doi: 10.1210/jcem.82.10.4269. [DOI] [PubMed] [Google Scholar]
- 45.Riedel C, Levy O, Carrasco N. Post-transcriptional regulation of the sodium/iodide symporter by thyrotropin. J Biol Chem. 2001;276:21458–63. doi: 10.1074/jbc.M100561200. [DOI] [PubMed] [Google Scholar]


