Abstract
We discussed the diagnostic and treatment value and clinical significance of computer assisted surgery system (Higemi) in precision surgeries for pediatric complex liver tumors. A total of 21 pediatric cases receiving hepatectomy for tumors in the portal vein and giant liver tumors from June 2012 to January 2015 were analyzed. Higemi was used for 3-dimensional (3D) reconstruction of thin-slice CT images and surgical planning. Tumors were precisely located and blood vessel neighborhood was determined so as to evaluate surgical feasibility. In addition, pathological classification, surgical time, intraoperative blood loss, transfusion rate and complications were predicted. After 3D reconstruction using Higemi, the neighboring relationship of tumors with blood vessels and the running direction of the blood vessels were clearly visualized. Of 21 cases, 10 cases had tumors located in the left lobe, 5 cases in the right lobe, 3 cases showing involvement of right trilobes, and 3 cases in the middle lobe. Lobes exceeding one third of the total liver volume were resected in 18 cases. Postoperative pathological examination indicated 10 cases of hepatoblastoma, 3 cases of hepatocellular carcinoma, 3 cases of hamartoma, 3 cases of infantile hemangioendothelioma, 1 case of teratoma and 1 case of undifferentiated malignant mesenchymoma. The surgical time was 90-240 min with an average of 130 min; the medium intraoperative blood loss was 60 ml and the minimum blood loss was 3 ml; the transfusion rate was 42.9% (9/21). Surgeries were successful in 20 cases, who were discharged after recovery. However, one case had giant liver tumor combined with severe obstructive jaundice and hepatic insufficiency and died of postoperative liver failure and DIC. 3D reconstruction of CT data using Higemi can clearly visualize the running direction of blood vessels and the neighboring relationship with tumors. Higemi can improve the precision and safety of complex hepatectomy.
Keywords: Surgery, computer assisted, liver resection, imaging, 3D
Introduction
The preferred therapy for pediatric liver tumors is radical resection. However, surgery is very difficult for pediatric cases due to delicate and complex anatomy of the liver, fast tumor growth, diversity of pathological types, and great differences in liver volume among cases of different age. It is highly important to accurately determine the position of the tumor, scope of involvement and the neighboring relationship with the blood vessels. Higemi [Hisense Gemini 3-dimensional (3D) Medical Imaging Reconstruction and Computer Assisted Surgery System] [1] jointly developed by Affiliated Hospital of Qingdao University Medical College and Hisense Group was used for 3D reconstruction of 64-row spiral CT images. The results of CT image 3D reconstruction were then applied to guide the precision surgery preoperatively and intraoperatively.
Materials and methods
Clinical data
The subjects were pediatric cases receiving hepatectomy for tumors in the portal vein and giant liver tumors from June 2012 to January 2014 with the assistance of Higemi, which included 9 males and 12 males aged 3.7 years on average (18 days-10 years). All cases were examined preoperatively by 64-row thin-slice contrast-enhanced CT scan. Imaging results indicated space-occupying lesions of the liver, which were located in the left lobe in 10 cases, in right lobe in 5 cases, as involvement of right trilobe in 3 cases, and in the middle lobe in 3 cases (Table 1).
Table 1.
The clinical characteristics of participants
| Characteristics | Data |
|---|---|
| Age (Year) | 3.7±4.4 |
| Sex (M/F) | 9/12 |
| Location (n, %) | |
| Left lobe (n, %) | 10 (47.6) |
| Middle lobe (n, %) | 3 (14.3) |
| Right trilobe (n, %) | 5 (23.8) |
| Right trilobe (n, %) | 3 (14.3) |
CT scanner and imaging parameters
The 64-row spiral CT scanner was used for thin-slice contrast-enhanced scanning (GE DISCOVERY CT750 HD, USA). Scanning parameters were as follows: tube voltage 120 kv, current 100 mAs, 0.625×64 detector array, slice thickness 5 mm, spacing 5 mm, collimation 40 mm, table feed speed, 27.5 mm/rot, speed of rotating frame 0.5 s/r, rotation speed of bulb tube 0.5 rot/s, matrix 512×512, delay time for arterial phase scan 20 s, delay time for portal venous phase scan 50 s. After scanning, the original images were uploaded to the workstation where they were stored.
Higemi
Higemi was jointly developed under the sponsorship from Key Projects in the National Science & Technology Pillar Program during the Twelfth Five-year Plan Period (2013BAI01B03). CT images were imported into Higemi, and the information of liver, spleen, pancreas, lesions and blood vessels was extracted. Through re-construction, a 3D rotating transparent model was obtained. Different colors were assigned to liver, pancreas, spleen, tumors, hepatic artery, hepatic vein, portal vein and inferior vena cava to make them more prominent (Figure 1).
Figure 1.
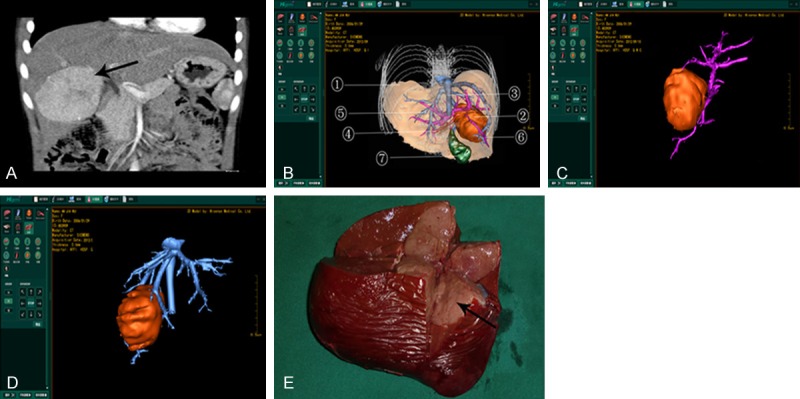
3D reconstruction using Higemi and 2D CT images. A. 3D reconstruction using Higemi clearly visualizes liver, gall bladder, pancreas, liver, tumor and neighboring blood vessels; B. 2D CT images show liver, gall bladder, pancreas and spleen.
Research method
Spiral CT scan
The cases were fasted for 4 h on the day of scanning. Venous access was established via the back of hand or forearm. For non-compliance cases, 10% chloral hydrate (0.5 ml/kg) was administered. Before scanning, 100-150 ml of 1%-2% Urografin was orally taken to set up negative control. The upper abdomen was scanned conventionally using 300 mg/ml non-ionic contrast medium Ultravist (1.5-2.0 ml/kg, no more than 2.0 ml/kg) at the injection rate of 1.0-2.5 ml/s. In the supine position, the region from the top of diaphgram to the lower margin of pancreas was scanned.
Steps of 3D reconstruction
CT images in Dicom format of three phases were imported into Higemi and reconstructed: Extraction of liver. Seed points for liver segmentation were selected in the cross-sectional view. Through drag-and-drop on the sagittal plane, the No. of CT images in the cross-sectional view was adjusted. With selection of several seed points, fast segmentation was implemented and the results were shown in 3D window; Extraction of tumor. Closed curves were drawn in the tumor area in cross-sectional view as markings. Then the tumor was segmented on coronal plane and sagittal plane and the 3D view of the tumor was generated; Extraction of information in intrahepatic blood vessels, including hepatic artery, portal vein, hepatic vein and inferior vena cava. The scope for reconstruction of blood vessels was delineated using the markers in the intrahepatic blood vessels. Adjustments were made to enhance sensitivity; Integration. Different colors were assigned to liver, tumor, hepatic artery, hepatic vein and portal vein. Transparency was adjusted for a more vivid 3D rendering (Figure 2).
Figure 2.
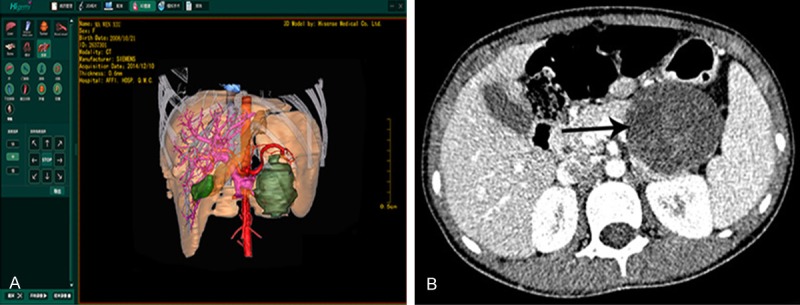
Medical records of one case with tumor in the middle lobe of liver. A. The arrow in ordinary contrast-enhanced CT image indicates a tumor in the middle lobe; B. 3D reconstructed image using multiplanar reconstruction (MPR) shows the rough position of tumor. However, the precise anatomical relationship between tumor, portal vein and its branches, hepatic artery and hepatic vein could not be visualized. The success rate of surgery was predicted only approximately; C. 3D reconstructed image using Higemi clearly shows the boundaries of tumor and the neighboring relationship with blood vessels: ① liver, ② tumor, ③ inferior vena cava, ④ hepatic artery, ⑤ hepatic vein, ⑥ portal vein, ⑦ gall bladder; D. The arrow indicates the tumor in the middle lobe in intraoperative finding; E. Gross specimen of tumor, pathologically classified as malignant mesenchymoma with high malignancy and low differentiation.
Preoperative planning
The boundaries of the lesions were determined, with delineation of area of resection and discrimination of normal tissues. Then the blood vessels and intrahepatic bile ducts to be severed were determined. The ducts that might be injured were predicted so as to avoid the risk of intraoperative hemorrhage. Appropriate approach of hepatectomy was chosen and anatomic liver resection was simulated using virtual reality technique. Before surgery, liver volume to be resected, tumor volume, and remnant functional liver function were calculated using Higemi. Surgical feasibility was evaluated by safety limit of liver resection.
Surgical approach
Venous access was established with close monitoring of vital signs. Ligaments around the liver were dissociated and the first porta hepatis was precisely anatomized according to surgical planning. Ligation and severing were performed for the hepatic artery branch, hepatic venous branch and common hepatic duct of the lobes to be resected. For right trisegmentectomy, right trisegmentectomy and right hemihepatectomy, the anatomical relationship between tumor and the third hepatic porta was dealt carefully. If the short hepatic vein was directly connected to the tumor or the short hepatic vein was very thick, anatomical separation and ligation of the short hepatic vein were performed carefully. Then the second hepatic porta was anatomized, with precise separation of the site where hepatic vein entered vena cava and ligation and severing of the hepatic vein. Finally a line was made with an electrotome. The tumor was resected using Cavitron Ultrasonic Surgical Aspirator (CUSA) and ligation was done with blood vessel forceps. Intrahepatic blood vessels and bile ducts were carefully ligated. Portal vein occlusion technique was adopted if it was necessary to reduce bleeding, but the duration should not exceed 20 min [2].
Results
Results of 3D reconstruction using Higemi
3D rendering of the livers and intrahepatic and extrahepatic blood vessels using Higemi allowed a clear visualization of the positions of liver and tumor, blood supply, relationship with canalis haemalis, running direction of intrahepatic blood vessels and their branches. Spatial configuration and abnormalities of these blood vessels could be observed by zooming in, zooming out, transparency adjustment and all-around rotation. The anatomical relationship was made prominent from various angles between lesions and intrahepatic blood vessels. The results of preoperative 3D reconstruction agreed well with intraoperative findings (Figure 3).
Figure 3.
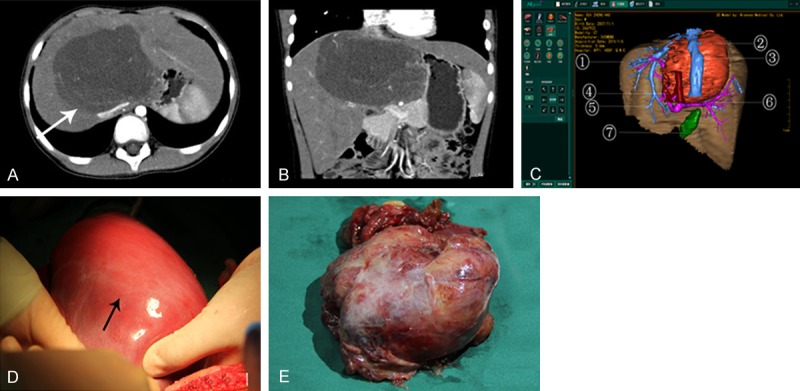
Medical records of one case with tumor in the right lobe. A. The arrow in the 2D CT image indicates tumor in the right lobe; B. 3D reconstructed image using Higemi clearly visualizes the boundaries of tumor and the neighboring blood vessels: ① liver, ② tumor, ③ inferior vena cava, ④ hepatic artery, ⑤ hepatic vein, ⑥ portal vein, ⑦ gall bladder; C. 3D reconstructed image using Higemi shows the anatomical relationship between tumor and portal vein and non-involvement of main trunk of the portal vein; D. 3D reconstructed image using Higemi displays the relationship between tumor and hepatic vein, with involvement of right hepatic vein; E. The arrow indicates tumor in intraoperative finding, which was pathologically confirmed as hepatoblastoma.
Surgical outcomes and pathological types
Among 21 cases, 18 cases had over one third of total liver volume resected. The minimum resection volume was 25%, and the maximum exceeded the volume of normal liver in children of the same age. Ten cases had tumor in the left lobe, including 3 cases receiving left lateral hepatic lobectomy (segment II+III) and 7 cases receiving left hemihepatectomy (segment II, III, IV, I). Five cases had tumors in the right lobe, including 3 cases receiving resection of segment VI+VII and 2 cases receiving resection of segment VIII. Tumors occurred in right lobes+left medial lobe in 3 cases and right trisegmentectomy was adopted (right anterior lobe+right posterior lobe+left medial lobe) at segment IV, V, VI, VII and VIII. Tumors occurred in right anterior lobe and left medial lobe in 3 cases, and resection of the middle lobe (right anterior lobe+left lateral lobe) was performed at segment IV, V and VIII. According to postoperative pathological classification, 10 cases were of hepatoblastoma, 3 cases of hepatocellular carcinoma, 3 cases of hamartoma, 3 cases of infantile hemangioendothelioma, 1 case of teratoma, and 1 case of undifferentiated malignant mesenchymoma.
Treatment effect and prognosis
Surgery was successful in 21 cases who showed stable intraoperative vital signs and normal hemodynamic indicators. Surgical time was 90-240 min with an average of 130 min. The medium intraoperative blood loss was 60 ml, and the minimum was 3 ml, with a transfusion rate of 42.9% (9/21). Twenty cases showed intestinal function recovery 1-2 d after surgery, with no occurrence of postoperative bile leakage, intra-abdominal bleeding, bowel obstruction and liver failure. The cases were discharged 7-14 d after surgery, showing good remnant liver function. One neonate died with giant space-occupying lesion in the right lobe. The tumor size was 290 ml as opposed to total liver volume of about 220 ml in normal neonates. Preoperative liver function test showed: TBIL 321.9 μmol/L, DBIL 215.10 μmol/L, IBIL 106.80 μmol/L, ALT 185.00 U/L, AST 231.00 U/L. It was judged by preoperative 3D reconstruction that the tumor was completely respectable. Portal vein occlusion technique was adopted if necessary. Intrahepatic blood vessels and bile ducts were carefully ligated and the tumor was completely resected. But the neonate died of severe hepatic insufficiency combined with DIC. Pathological classification of this case was hepatoblastoma of embryonic type.
Discussion
The incidence of pediatric malignancies is lower than that of adult malignancies with a variation of tumor types. As severe infectious diseases that once endangered children’s life were reduced, malignancies now become the leading cause of death in children [2,3].
Pediatric liver tumors should be treated comprehensively, and radical surgery is still the most important option [4,5]. Computer assisted surgery system is also the main topic of Key Projects in the National Science & Technology Pillar Program during the Twelfth Five-year Plan Period. Supported by this Program, the present study discussed the clinical application and significance of computer assisted surgery system in pediatric complex liver tumors.
For conventional surgeries, the 3D configuration is reproduced in the surgeon’s mind based on anatomical knowledge, experience, and 2D images from B-mode ultrasound and CT scan. It is sometimes impossible to determine the final surgical scheme until exploratory laparotomy [6-8]. As a result, great uncertainties arise from 2D preoperative planning in the aspects of preoperative planning, surgical procedures and postoperative recovery. Specifically, scope of tumor invasion, blood supply, neighboring relationship with surrounding blood vessels, running direction of intrahepatic blood vessels, resectability of tumor, resection scope and method cannot be determined with full certainty before surgery.
Precision hepatectomy is an emerging technique in hepatobiliary surgery based on the principles of maximum lesion removal, maximum preservation of normal tissues, minimal invasiveness and reduction of intraoperative bleeding. It is an approach to achieve surgical safety, high effectiveness and minimal invasiveness simultaneously [9]. We used Higemi for 3D reconstruction of CT images so as to visualize tumor size, tumor location, blood supply and neighboring relationship with the blood vessels. This reconstruction system can accurately find the anatomical variations of intrahepatic blood vessels in pediatric liver tumors [10] and allow preoperative assessment and planning. Using this method, surgical safety and controllability can be improved [11,12], and individualized surgery is possible. Through virtual reality technique, comparison and optimization among the schemes can be done, thereby avoiding unnecessary exploratory laparotomy and extending the indications for liver tumor resection. A revision is usually made using 3D rendering on the basis of 2D preoperative planning [13,14]. For one typical case with tumor in middle lobe (Figure 2, undifferentiated malignant mesenchymoma), 3D rendering showed that the giant tumor was enveloped by the left and right branches of portal vein and the left, middle and right hepatic veins. Thus the surgeon was provided with precise clues for surgery. For another case with tumor in the right lobe (Figure 3, hepatoblastoma), the position of tumor and the neighboring relationship with the blood vessels could be hardly determined by 2D CT scan. With Higemi the tumor was precisely located and different colors were assigned to each important blood vessel. It was found that the main trunk of the portal vein was not involved, but the right branch of the portal vein was affected, so right hepatic lobectomy was performed.
However, one neonate died with giant, fast-growing tumor and hepatic insufficiency. We suspected that severe obstructive jaundice was caused by compression of the tumor. Any delay of surgery would aggravate hepatic injury and endanger the life of the neonate. The relatives of the neonate only required one-time surgical resection. The giant tumor was resected based on results of 3D reconstruction, but the neonate had postoperative DIC and died. The resection of giant liver tumor with portal vein involvement or liver tumor primary to porta hepatis usually has a high risk. The difficulty exists in the choice between two schemes: one-stage resection, or chemotherapy followed by second-stage resection after achieving tumor shrinkage. For confirmed hepatoblastoma, chemotherapy can obviously reduce tumor size and surgical risk. However, if the diagnosis is wrong, chemotherapy and chemoembolization will only make things worse. Laparotomy or laparoscopy will be recommended to obtain biopsies if the anticipated outcomes of one-stage resection are poor. Then chemotherapy followed by surgery may be considered based on biopsy results. At present, chemotherapy in neonates is still controversial. In China, many parents demand one-stage resection only. Given the current social situations of China, medical disputes may arise if chemotherapy is administered without pathological confirmation in spite of highly suspicious imaging findings and positive AFP test. Now the relationship between preoperative chemotherapy, estimate of remnant liver volume and surgical efficacy and the significance of computer assisted surgery system in large case series are the major concerns in Key Projects in the National Science & Technology Pillar Program during the Twelfth Five-year Plan Period.
Advances in digital medicine facilitate the transition from conventional surgery to precision surgery for liver tumors [15]. Computer assisted surgery system offers an important tool for diagnosis of pediatric liver tumors and greatly benefits preoperative planning and intraoperative navigation. Surgical safety and efficacy are improved using computer assisted surgery system.
Acknowledgements
This work was supported by the National “Twelfth Five-Year” Plan for Science & Technology Support of China (Grant No. 2013BAI01B03); the Major Science & Technology Project of Independent Innovation of Qingdao (Grant No. 14-6-1-6-zdzx).
Disclosure of conflict of interest
None.
References
- 1.Dong Q, Chen YJ, Lu Y, Wang GD, Xu WJ, Pan ZK, Gao C. Development and clinical application of digital medicine and computer aided surgery. e-Healthcare. 2013;9:58–61. [Google Scholar]
- 2.Dong Q. Pediatric tumor surgery. Beijing: People’s Health Publishing House; pp. 508–546. [Google Scholar]
- 3.Dong Q, Wang BL. Diagnosis and treatment of liver tumor in children and computer aided liver resection. J Clin Surg. 2013;21:585–587. [Google Scholar]
- 4.Dong Q, Jiang B, Lu Y, Zhang H, Jiang Z, Lu H, Yang C, Zhao J, Hao X. Surgical management of giant liver tumor involving the hepatic hilum of children. World J Surg. 2009;33:1520–1525. doi: 10.1007/s00268-009-0060-0. [DOI] [PubMed] [Google Scholar]
- 5.Jin SG, Zhong L, Xiang B, Li FY, Jiang XP, Xu ZC. Precise liver resection for giant pediatric hepatic neoplasm: a report of 30 cases. Chin J Pediatr Surg. 2013;34:262–265. [Google Scholar]
- 6.Wakabayashi H, Lshimura K, Izuishi K, Karasawa Y, Maeta H. Evaluation of liver function for hepatic resection for hepatocellular carcinoma in the liver with damaged parenchyma. J Surg Res. 2003;116:248–252. doi: 10.1016/j.jss.2003.09.015. [DOI] [PubMed] [Google Scholar]
- 7.Wang X, Li J, Wang H, Luo Y, Ji W, Duan W, Zhang X, Guo S, Xu K, Dong J, Zheng S. Validation of the laparoscopically stapled approach as a standard technique for left lateral segment liver resection. World J Surg. 2013;37:806–811. doi: 10.1007/s00268-013-1912-1. [DOI] [PubMed] [Google Scholar]
- 8.Dong S, Jiang BX, Zhang H, Jiang Z, Lu HT, Xu WJ, Yang XD, Hao XW. Use of three-dimensional computerized tomography reconstruction in giant liver tumors in children. Chin J Pediatr Surg. 2006;27:6–9. [Google Scholar]
- 9.Dong JH, Huang ZQ. To advocate precise hepatectomy and recreate the legend of Prometheus. Chin J Digest Surg. 2010;9:4–5. [Google Scholar]
- 10.Yamanaka J, Saito S, Fujimoto J. Impact of preoperative planning using virtual segmental volumetry on liver resection for hepatocellular carcinoma. World J Surg. 2007;31:1249–1255. doi: 10.1007/s00268-007-9020-8. [DOI] [PubMed] [Google Scholar]
- 11.Dong Q, Xu W, Jiang B, Lu Y, Hao X, Zhang H, Jiang Z, Lu H, Yang C, Cheng Y, Yang X, Hao D. Clinical applications of computerized tomography 3-D reconstruction imaging for diagnosis and surgery in children with large liver tumors or tumors at the hepatichilum. Pediatr Surg Int. 2007;23:1045–1050. doi: 10.1007/s00383-007-1910-1. [DOI] [PubMed] [Google Scholar]
- 12.Zhao J, Dong Q, Jiang BX, Zhang H, Jiang Z, Lu HT, Niu HT, Hao XW. Application of three-dimensional computerized tomography reconstruction and hepatic volume measurement in liver tumor operation in children. J Clin Pediatr Surg. 2009;8:13–16. [Google Scholar]
- 13.Fang CH, You JH, Lau WY, Lai EC, Fan YF, Zhong SZ, Li KX, Chen ZX, Su ZH, Bao SS. Anatomical variations of hepatic veins: three-dimensional computed tomography scans of 200 subjects. World J Surg. 2012;36:120–124. doi: 10.1007/s00268-011-1297-y. [DOI] [PubMed] [Google Scholar]
- 14.Fang CH, Huang YP, Chen ML, Lu CM, Li XF, Qiu WF. Digital medical technology based on 64-slice computed tomography in hepatic surgery. Chin Med J (Engl) 2010;123:1149–1153. [PubMed] [Google Scholar]
- 15.Fang SH. The developing situation and Prospect of digital medicine in China. J Pract Med. 2014;30:169–171. [Google Scholar]


