Abstract
Objective: This study was to analyze the acylation-stimulating protein (ASP) (301T>C) and C5a-like receptor 2 (C5L2) (698C>T) gene polymorphisms in Han and Hui populations, and investigate their association with coronary heart disease (CHD). Methods: 245 Han CHD patients and 110 Hui CHD patients from Shandong, Jinan, China were included in this study. Biochemical analysis was performed to assess the blood sugar and lipid levels in these patients, and the TaqMan genotyping assay was used to determine the genotype distribution. Results: Our results showed that the C allele frequency in the ASP (301T>C) polymorphism in the Hui population was significantly higher than normal controls, while no significant differences were observed in the Han population, which might contribute to the genetic susceptibility of CHD in the Hui population. Moreover, for C5L2 (698C>T) gene polymorphism in both Han and Hui populations, the frequencies of the C/T genotype and T allele were significantly higher in the CHD patients compared with normal controls. Moreover, there were slight differences in the association of ASP and C5L2 gene polymorphisms with blood sugar and lipid levels between Han and Hui populations. Conclusions: Our results suggest differential ASP and C5L2 genotype distributions between Han and Hui patients, which might be associated with the different CHD-related genetic susceptibilities in these populations. These findings might contribute to a better understanding of the etiology and pathogenesis of CHD in different regions and populations.
Keywords: Acylation-stimulating protein (ASP), C5a-like receptor 2 (C5L2), gene polymorphism, coronary heart disease
Introduction
Epidemiological studies show that the incidence of coronary heart disease (CHD) is associated with environmental and genetic factors [1]. Sugar and lipid metabolism disorders, such as increased triglyceride (TG) and fasting blood sugar levels, have been recognized as major risk factors for CHD [2,3]. Therefore, sugar and lipid metabolism-related genes might also be involved in CHD pathogenesis, such as acylation-stimulating protein (ASP) and its specific receptor, C5a-like receptor 2 (C5L2) [4].
ASP, also known as C3a des-Arg, stimulates TG synthesis [5] and sugar transportation [6]. ASP is the rate-limiting enzyme in the catabolism of TG-rich lipoprotein, which could elevate serum TG level as a non-competitive inhibitor of lipoprotein lipase [7]. ASP has also been shown to stimulate TG storage in adipose tissues [8]. C5L2, a G protein-coupled receptor, is the functional receptor of ASP [9]. Active C5L2 initiates downstream signaling transduction pathways, including protein kinase C activation and sugar transporter translocation [10]. The cascade amplification increases sugar transportation and fatty acid esterification, leading to the accumulation of TG. Therefore, C5L2 activity may influence the body’s susceptibility to CHD. In recent years, ASP and C5L2 gene polymorphisms have been intensively investigated, especially concerning their association with type 2 diabetes and lipid metabolism disorders. However, the relationship between the polymorphism of ASP and C5L2 genes and CHD has not yet been fully understood.
In this study, ASP and C5L2 gene polymorphisms in Han and Hui populations from Shandong, Jinan, China were analyzed and compared, and their association with CHD was also investigated. Our findings might contribute to the understanding of etiology and pathogenesis of CHD in different regions and populations.
Materials and methods
Patients
This study included 245 Han CHD patients (125 males and 120 females), and 110 Hui CHD patients (58 males and 52 females), who were admitted to Provincial Hospital affiliated to Shandong University from Jan 2010 to Dec 2013. These patients were 25-80 years old, and at least one of the following inclusion criteria should be met: (1) at least one coronary diameter stenosis of ≥ 50%, demonstrated by coronary angiography; (2) diagnosis of acute myocardial infarction (with clinical symptoms of ischemic chest pain and/or discomfort; dynamic evolution of ECG indicative of acute myocardial infarction, i.e., ST-segment elevation or emerging left bundle-branch block; cardiac troponin T (cTnT) or creatine kinase (CK-MB) levels higher than two times the normal upper limit, with dynamic changes); (3) diagnosis of old myocardial infarction (with a previous history of acute myocardial infarction; pathological Q waves in ECG; regional myocardial loss confirmed by echocardiography or cardiac ECT). On the other hand, patients with (1) liver and/or kidney dysfunction; (2) concurrent infection, cancers, or immune system disorders; (3) cerebrovascular diseases; (4) coagulation dysfunction; or (5) peripheral vascular diseases were excluded from the study. Another 250 Han (127 males and 123 females) and 150 Hui (80 males and 70 females) subjects with normal coronary angiography and CT angiography, as well as normal results from ECG, echocardiography, and cardiac ECT, were used as normal control subjects. There were no significant differences in gender, age, and other general information among these subjects. Prior written and informed consent were obtained from every patient and the study was approved by the ethics review board of Provincial Hospital affiliated to Shandong University.
Biochemical analysis
Serum concentrations of total cholesterol (TC), total triglyceride (TG), high-density lipoprotein cholesterol (HDL-C), LDL-C, sugar, and other indicators were measured using standard methods in the Central Laboratory of Provincial Hospital affiliated to Shandong University.
TaqMan genotyping assay
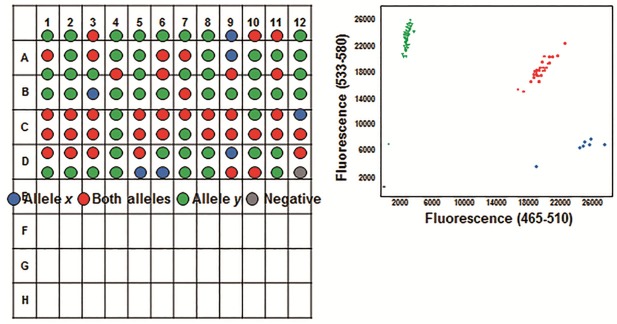
Three millilitres of fasting venous blood was collected in EDTA tubes, and genomic DNA was isolated with the RelaxGene Blood DNA System (TianGen Biotech, Beijing, China), according to the manufacture’s instructions. TaqMan genotyping assay was performed using an ABI ViiA7 machine (Applied Biosystems, Foster City, CA, USA) with the Endpoint Genotyping method (Figure 1). Briefly, two TaqMan probes were used that differed at the polymorphic sites: one probe was complementary to the wild-type allele (labeled with FAM fluorescence reporter) and the other to the variant allele (labeled with VIC fluorescence reporter). The reporter dye and the quencher dye are covalently linked to the wild-type or variant allele TaqMan probes. When the probes were intact, fluorescence of the reporter dye would be quenched by the quencher dye. During the PCR annealing steps, TaqMan probes hybridized to targeted polymorphic sites. During the extension phase, the reporter dye is cleaved by the Taq polymerase, resulting in an increased fluorescence of the specific reporter dye. The genotyping was determined by detecting the fluorescence intensity of the two different reporter dyes after PCR (Figure 2). Polymerase chain reaction (PCR) was carried out with 80-100 ng genomic DNA in a 10 μL reaction system. TaqMan-PCR program consisted of an initial denaturation step at 95°C for 10 min, and then 35 cycles of 95°C for 10 s, 60°C for 30 s, and 72°C for 1 min, followed by a final cooling step at 40°C for 30 s.
Figure 1.
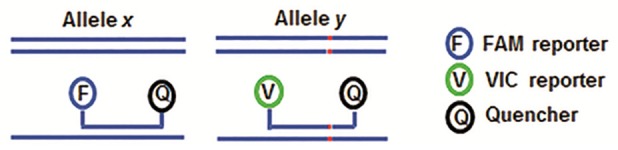
TaqMan genotyping assay. Two TaqMan probes with different polymorphic sites were labeled with different reporter dyes (FAM and VIC). Only the hybridized probe would be cleaved by the Taq polymerase during PCR extension phase. After PCR, the genotyping was determined by measuring the intensity distribution of the two different dyes.
Figure 2.
Typical results for the Endpoint Genotyping analysis. The blue, red, and green fluorescence indicated the homozygous X/X, heterozygous X/Y, and homozygous Y/Y genotypes, respectively.
Statistical analysis
Data were expressed as mean ± SD. SPSS16.0 software was used for statistical analysis. Hardy-Weinberg equilibrium was assessed by X2 analysis. Continuous variables were compared using the t-test and analysis of variance. Analysis of covariance was used for the comparison of blood sugar and lipid levels between different genotypes. P < 0.05 was considered statistically significant.
Results
Participant characteristics
General characteristics of CHD patients and normal control subjects were shown in Table 1. Significant differences in the following variables were observed between CHD patients and normal control subjects: waistline body mass index (BMI), systolic blood pressure (SBP), diastolic blood pressure (DBP), as well as serum concentrations of TC, LDL-C, TG, FBG, ASP, and C3 (all P < 0.05). On the other hand, there were no significant differences in serum HDL-C level between CHD patients and normal control subjects. In the following sections, genotype distributions and allele frequencies of the polymorphic sites in ASP and C5L2 genes in these Han and Hui populations were analyzed and compared.
Table 1.
Characteristics of the participants
| CHD patients | Normal controls | |
|---|---|---|
| Waistline, cm | 96.41±6.78* | 83.45±4.58 |
| BMI, kg/m2 | 28.01±2.31* | 24.04±2.46 |
| SBP, mmHg | 157.96±11.73* | 125.02±6.92 |
| DBP, mmHg | 89.07±9.46* | 78.32±6.15 |
| TC, mmol/L | 5.14±1.17* | 4.43±0.89 |
| HDL-C, mmol/L | 1.31±0.34 | 1.45±0.67 |
| LDL-C, mmol/L | 2.73±1.18* | 2.14±0.59 |
| TG, mmol/L | 1.97±0.99* | 1.33±0.77 |
| FBG, mmol/L | 6.34±0.67* | 5.18±0.52 |
| ASP, µg/L | 5.97±1.33* | 4.89±1.13 |
| C3, µg/ml | 707.6±215.7* | 589.4±115.6 |
Abbreviations: BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; TC, total cholesterol; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; TG, total triglyceride; FBG, fasting blood glucose; ASP, acylation-stimulating protein. Note: Compared with the control group;
P < 0.05.
Genotype distributions and allele frequencies of polymorphic sites in ASP and C5L2 genes in CHD patients and normal controls
Genotype and allele distributions of the ASP (301T>C) and C5L2 (698C>T) gene polymorphic sites in the study population were shown in Tables 2 and 3, respectively. Genotype distribution of ASP (301T>C) did not exhibit significant difference from the Hardy-Weinberg equilibrium expectations in both populations (X2 = 0.885, P = 0.35 for Han population, and X2 = 0.077, P = 0.78 for Hui population, respectively). Moreover, genotype distribution of C5L2 (698C>T) did not show significant difference from the Hardy-Weinberg equilibrium expectations in both populations, either (X2 = 0.873, P = 0.35 for Han population, and X2 = 1.449, P = 0.23 for Hui population, respectively).
Table 2.
Distribution of genotype and allele frequencies of polymorphic site of ASP in Han and Hui populations
| Group | N | Genotype (N, %) | χ2 | P | Allele frequency (N, %) | χ2 | P | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||||
| C/C | C/T | T/T | C | T | |||||||
| Han | Control | 250 | 18 (7.2) | 82 (32.8) | 150 (60.0) | 0.534 | > 0.05 | 118 (23.6) | 382 (76.4) | 0.032 | > 0.05 |
| CHD | 245 | 15 (6.1) | 88 (35.9) | 142 (58.0) | 118 (24.1) | 372 (75.9) | |||||
| Hui | Control | 150 | 15 (10.0) | 54 (36.0) | 81 (54.0) | 0.175 | < 0.05 | 84 (28.0) | 216 (72.0) | 0.230 | < 0.05 |
| CHD | 110 | 15 (13.6) | 44 (40.0) | 51 (46.4) | 74 (33.6) | 146 (66.4) | |||||
Table 3.
Distribution of genotype and allele frequencies of polymorphic site of C5L2 in Han and Hui populations
| Group | N | Genotype (N, %) | χ2 | P | Allele frequency (N, %) | χ2 | P | |||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||
| C/C | C/T | C | T | |||||||
| Han | Control | 250 | 235 (94.0) | 15 (6.0) | 9.355 | < 0.05 | 485 (97.0) | 15 (3.0) | 8.857 | < 0.05 |
| CHD | 245 | 210 (85.7) | 35 (14.3) | 455 (92.8) | 35 (7.2) | |||||
| Hui | Control | 150 | 144 (96.0) | 6 (4.0) | 11.578 | < 0.05 | 294 (98.0) | 6 (2.0) | 11.018 | < 0.05 |
| CHD | 110 | 92 (83.6) | 18 (16.4) | 202 (91.8) | 18 (8.2) | |||||
As shown in Table 2, in Han subjects, the genotype frequencies of C/C, C/T, and T/T in ASP (301T>C) polymorphism were 6.1%, 35.9%, and 58.0%, respectively, in the CHD group, while the genotype frequencies were 7.2%, 32.8%, and 60.0%, respectively, in the control group (P > 0.05). Moreover, the C allele frequencies in Han CHD patients and normal controls were 24.1% and 23.6%, respectively (P > 0.05). On the other hand, in the Hui population, the genotype frequencies of C/C, C/T, and T/T in ASP (301T>C) polymorphism were 13.6%, 40.0%, and 46.4%, respectively, which was significantly different from their normal control counterparts (C/C, C/T, and T/T frequencies of 10.0%, 36.0%, and 54.0%, respectively) (P < 0.05). The C allele frequency in the CHD group (25.5%) was significantly lower than the normal controls (27.3%) (P < 0.05).
For C5L2 (698C>T) gene polymorphism, as shown in Table 3, in the Han population, the genotype frequencies of C/C and C/T were 85.7% and 14.3%, respectively, in the CHD patients, while the genotype frequencies were 94.0% and 6.0%, respectively, in the control group (P < 0.05). Furthermore, the C allele frequencies in Han CHD patients was 92.8%, which was significantly lower than the normal controls (97.0%) (P < 0.05). In addition, for C5L2 (698C>T) gene polymorphism in the Hui population, the genotype frequencies of C/C and C/T were 83.6% and 16.4%, respectively, in the CHD group, which was significantly different from the Hui control group (the C/C and C/T genotype frequencies of 96.0% and 4.0%, respectively) (P < 0.05). Furthermore, the C allele frequencies for C5L2 (698C>T) gene polymorphism in Hui CHD patients was 91.8%, which was significantly lower than the control group (98.0%) (P < 0.05). Taken together, these results suggest that the C allele frequency in ASP (301T>C) polymorphism in the Hui population is significantly higher than normal controls, while no significant differences were observed in the Han population, which might contribute to the genetic susceptibility of CHD in the Hui population. Moreover, for C5L2 (698C>T) gene polymorphism in both Han and Hui populations, the frequencies of CT genotype and T allele are significantly higher in the CHD patients compared with normal controls.
Association of ASP and C5L2 gene polymorphisms with blood sugar and lipid levels in Han and Hui populations
To further investigate the functional role of ASP and C5L2 gene polymorphisms, the blood sugar and lipid levels between the different genotype carriers in Han and Hui populations were assessed. As shown in Tables 4 and 5, in the Han population, ASP C/C and C5L2 C/T genotypes were both associated with the blood sugar level. However, no association was observed between these genotypes and blood lipid levels. On the other hand, in the Hui population, ASP C/C and C5L2 C/T genotypes were also associated with the blood sugar level. Furthermore, for the blood lipid levels, ASP C/C and C5L2 C/T genotypes were associated with the blood TG level, while these genotypes were not associated with serum concentrations of TC, HDL-C, or LDL-C. These results suggest that there are slight differences in the association of ASP and C5L2 gene polymorphisms with blood sugar and lipid levels between Han and Hui populations.
Table 4.
Association of ASP gene polymorphisms with blood sugar and lipid levels in Han and Hui populations
| Group | Genotype (N, %) | t | P | ||
|---|---|---|---|---|---|
|
| |||||
| C/C | C/T+T/T | ||||
| Han | TG (mmol/L) | 2.2±0.6 | 2.0±0.8 | 0.9502 | > 0.05 |
| TC (mmol/L) | 5.2±1.3 | 5.4±1.1 | -2.1477 | > 0.05 | |
| DHL-C (mmol/L) | 1.1±0.5 | 1.2±0.3 | -1.1913 | > 0.05 | |
| LDL-C (mmol/L) | 3.4±1.3 | 3.5±1.2 | -0.3112 | > 0.05 | |
| FBG (mmol/L) | 5.4±1.4 | 4.8±1.2 | 1.8571 | < 0.05 | |
| Hui | TG (mmol/L) | 2.3±0.4 | 1.9±0.7 | 1.6952 | < 0.05 |
| TC (mmol/L) | 5.3±1.3 | 5.2±1.2 | 0.2380 | > 0.05 | |
| DHL-C (mmol/L) | 1.3±0.6 | 1.3±0.3 | 0.0000 | > 0.05 | |
| LDL-C (mmol/L) | 3.5±1.2 | 3.4±1.4 | 0.2074 | > 0.05 | |
| FBG (mmol/L) | 5.2±1.5 | 4.7±1.1 | 1.2670 | < 0.05 | |
Table 5.
Association of C5L2 gene polymorphisms with blood sugar and lipid levels in Han and Hui populations
| Group | Genotype (N, %) | t | P | ||
|---|---|---|---|---|---|
|
| |||||
| C/C | C/T | ||||
| Han | TG (mmol/L) | 2.3±0.5 | 2.4±0.8 | 0.9925 | > 0.05 |
| TC (mmol/L) | 5.4±1.4 | 5.1±1.3 | 1.1852 | > 0.05 | |
| DHL-C (mmol/L) | 1.2±0.8 | 1.1±0.6 | 0.7066 | > 0.05 | |
| LDL-C (mmol/L) | 3.5±1.1 | 3.4±1.4 | 0.4776 | > 0.05 | |
| FBG (mmol/L) | 5.2±1.1 | 5.8±1.3 | -2.9080 | < 0.05 | |
| Hui | TG (mmol/L) | 2.4±0.8 | 2.0±1.0 | 1.8594 | < 0.05 |
| TC (mmol/L) | 5.4±1.5 | 5.3±1.6 | 0.2559 | > 0.05 | |
| DHL-C (mmol/L) | 1.1±0.6 | 1.2±0.4 | -0.6769 | > 0.05 | |
| LDL-C (mmol/L) | 3.3±1.4 | 3.4±1.3 | -0.2802 | > 0.05 | |
| FBG (mmol/L) | 5.1±1.4 | 5.7±1.5 | -1.6438 | < 0.05 | |
Discussion
ASP was discovered by Cianflones et al. [11] in 1989, which was a small molecule isolated from human plasma, consisting of 76 amino acid residues. It is an autocrine hormone derived from adipose cells, regulating the storage of adipose, as well as the TG synthesis and sugar transportation. ASP promotes postprandial TG clearance [12], and plays an important role in sugar and lipid metabolism. In fact, the biological role of ASP is closely linked with its receptor, C5L2. C5L2, a member of C5aR (CD88) subfamily, is widely distributed in most tissues and cells throughout the body. C5L2 is abundantly expressed in granulocytes and immature dendritic cells, as well as in the spleen. It has been confirmed that, ASP could bind to C5L2, making it a real functional receptor [13]. In addition, via binding to C5L2, ASP could initiate a series of events, including phosphorylation, β-arrestin translocation, and receptor internalization [14]. Furthermore, active C5L2 might activate downstream signaling pathways, such as the activation and translocation of protein kinase C and sugar transporters [15,16].
It has been widely confirmed that, the levels of ASP are significantly elevated in patients with CHD, diabetes, and obesity. A study from Yang et al. [17] has shown that, in the Han and Uygur populations from Xinjiang, China, ASP (301T>C) polymorphism might be associated with the pathogenesis of type 2 diabetes and dyslipidemia. Moreover, Zheng et al. [18,19] suggest that, in the Han and Uygur populations from Xinjiang, China, C5L2 (698C>T) and (901G>A) polymorphic sites might be related to the etiologies of CHD and diabetes.
In the present study, TaqMan genotyping was performed to investigate ASP (301T>C) and C5L2 (698C>T) polymorphisms in Han and Hui populations from Shandong, Jinan, China. According to our results, ASP (301T>C) polymorphic site had been found in the Hui population, and the C allele frequency was significantly higher in the CHD group compared with the control group, indicating that the C/C genotype may be associated with the CHD-related genetic susceptibility, as well as the blood sugar and TG levels, in this population. However, no significant differences were observed in the genotype distribution between the Han CHD patients and normal control subjects. ASP (301T>C) polymorphism was not associated with lipid metabolism, but could be involved in the sugar metabolism.
On the other hand, for the C5L2 (698C>T) polymorphism, the frequencies of heterozygous C/T genotype were higher in the CHD group than normal controls, in both Han and Hui populations, and the T allele frequencies were also higher in the CHD patients, for both Han and Hui populations. Moreover, the genotype distribution might be associated with the blood sugar and TG levels in the Hui population.
In conclusion, we first investigated the relationship between the ASP (301T>C) and C5L2 (698C>T) gene polymorphisms in Han and Hui populations from Shandong, Jinan, China. Our results indicated differential genotype distributions of ASP and C5L2 genes between Han and Hui populations, which might be associated with the CHD-related genetic susceptibilities in these populations. These results might contribute to a better understanding of the etiology and pathogenesis of CHD in different regions and populations.
Disclosure of conflict of interest
None.
References
- 1.Stumvoll M, Goldstein BJ, van Haeften TW. Type 2 diabetes: principles of pathogenesis and therapy. Lancet. 2005;365:1333–1346. doi: 10.1016/S0140-6736(05)61032-X. [DOI] [PubMed] [Google Scholar]
- 2.Arsenault BJ, Lemieux I, Després JP, Wareham NJ, Kastelein JJP, Khaw KT, Boekholdt SM. The hypertriglyceridemic-waist phenotype and the risk of coronary artery disease: results from the EPIC-Norfolk prospective population study. CMAJ. 2010;182:1427–1432. doi: 10.1503/cmaj.091276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goswami B, Rajappa M, Singh B, Ray PC, Kumar S, Mallika V. Inflammation and dyslipidaemia: a possible interplay between established risk factors in North Indian males with coronary artery disease. Cardiovasc J Afr. 2010;21:103–108. [PMC free article] [PubMed] [Google Scholar]
- 4.Chan JCN, Malik V, Jia W, Kadowaki T, Yajnik CS, Yoon KH, Hu FB. Diabetes in Asia: epidemiology, risk factors, and pathophysiology. JAMA. 2009;301:2129–2140. doi: 10.1001/jama.2009.726. [DOI] [PubMed] [Google Scholar]
- 5.Paglialunga S, Julien P, Tahiri Y, Cadelis F, Bergeron J, Gaudet D, Cianflone K. Lipoprotein lipase deficiency is associated with elevated acylation stimulating protein plasma levels. J Lipid Res. 2009;50:1109–1119. doi: 10.1194/jlr.M800430-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Lind van Wijngaarden RF, Cianflone K, Gao Y, Leunissen RW, Hokken-Koelega AC. Cardiovascular and metabolic risk profile and acylation-stimulating protein levels in children with Prader-Willi syndrome and effects of growth hormone treatment. J Clin Endocrinol Metab. 2010;95:1758–1766. doi: 10.1210/jc.2009-0656. [DOI] [PubMed] [Google Scholar]
- 7.Yasruel Z, Cianflone K, Sniderman AD, Rosenbloom M, Walsh M, Rodriguez MA. Effect of acylation stimulating protein on the triacylglycerol synthetic pathway of human adipose tissue. Lipids. 1991;26:495–499. doi: 10.1007/BF02536592. [DOI] [PubMed] [Google Scholar]
- 8.Chen Z, Zhang X, Ma G, Qian Q, Yao Y. Association study of four variants in KCNQ1 with type 2 diabetes mellitus and premature coronary artery disease in a Chinese population. Mol Biol Rep. 2010;37:207–212. doi: 10.1007/s11033-009-9597-0. [DOI] [PubMed] [Google Scholar]
- 9.Cui W, Simaan M, Laporte S, Lodge R, Cianflone K. C5a- and ASP-mediated C5L2 activation, endocytosis and recycling are lost in S323I-C5L2 mutation. Mol Immunol. 2009;46:3086–3098. doi: 10.1016/j.molimm.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 10.Maslowska M, Wang HW, Cianflone K. Novel roles for acylation stimulating protein/C3adesArg: a review of recent in vitro and in vivo evidence. Vitam Horm. 2005;70:309–332. doi: 10.1016/S0083-6729(05)70010-8. [DOI] [PubMed] [Google Scholar]
- 11.Cianflone KM, Sniderman AD, Walsh MJ, Vu HT, Gagnon J, Rodriguez MA. Purification and characterization of acylation stimulating protein. J Biol Chem. 1989;264:426–430. [PubMed] [Google Scholar]
- 12.Cianflone K, Maslowska M, Sniderman AD. Acylation stimulating protein (ASP), an adipocyte autocrine: new directions. Semin Cell Dev Biol. 1999;10:31–41. doi: 10.1006/scdb.1998.0272. [DOI] [PubMed] [Google Scholar]
- 13.Kalant D, Cain SA, Maslowska M, Sniderman AD, Cianflone K, Monk PN. The chemoattractant receptor-like protein C5L2 binds the C3a des-Arg77/acylation-stimulating protein. J Biol Chem. 2003;278:11123–11129. doi: 10.1074/jbc.M206169200. [DOI] [PubMed] [Google Scholar]
- 14.Huang Y, Xu M, Hong J, Gu W, Bi Y, Li X. -607 C/A polymorphism in the promoter of IL-18 gene is associated with 2 h post-loading plasma glucose level in Chinese. Endocrine. 2010;37:507–512. doi: 10.1007/s12020-010-9338-0. [DOI] [PubMed] [Google Scholar]
- 15.Karadeniz M, Erdogan M, Cetinkalp S, Berdeli A, Eroglu Z, Ozgen AG. Monocyte chemoattractant protein-1 (MCP-1) 2518G/A gene polymorphism in Turkish type 2 diabetes patients with nephropathy. Endocrine. 2010;37:513–517. doi: 10.1007/s12020-010-9342-4. [DOI] [PubMed] [Google Scholar]
- 16.Marcil M, Vu H, Cui W, Dastani Z, Engert JC, Gaudet D, Castro-Cabezas M, Sniderman AD, Genest J, Cianflone K. Identification of a novel C5L2 variant (S323I) in a French Canadian family with familial combined hyperlipemia. Arterioscler Thromb Vasc Bio. 2006;26:1619–1625. doi: 10.1161/01.ATV.0000222907.72985.0b. [DOI] [PubMed] [Google Scholar]
- 17.Chen Y, Ma YT. Letter to the Editor Reply to commentary by A. Fisette on the article “Relationship between the acylation-stimulating protein gene and coronary heart disease in the Xinjiang Uygur and Han populations of China” published in Genetics and Molecular Research Genetics and molecular research. GMR. 2014;13:9136–9137. doi: 10.4238/2014.November.3.2. [DOI] [PubMed] [Google Scholar]
- 18.Alharbi KK, Khan IA, Syed R. Circulating C5L2 gene polymorphism is associated with type 2 diabetes mellitus in Saudi population. Mol Biol Rep. 2013;40:6323–6327. doi: 10.1007/s11033-013-2745-6. [DOI] [PubMed] [Google Scholar]
- 19.Zheng YY, Xie X, Ma YT, Yang YN, Fu ZY, Li XM, Ma X, Chen BD, Liu F. A novel polymorphism (901G > a) of C5L2 gene is associated with coronary artery disease in Chinese Han and Uyghur population. Lipids Health Dis. 2013;12:139. doi: 10.1186/1476-511X-12-139. [DOI] [PMC free article] [PubMed] [Google Scholar]