Abstract
Objective: We analyzed the correlation between mutation in intron 4 and exon 7 of endothelial nitric oxide synthase (eNOS) and avascular necrosis of femoral head (ANFH). Method: A total of 260 ANFH cases without history of hip joint injuries were diagnosed and subject to staging according to Ficat standard, with 262 health subjects as control. Venous blood was collected to extract genome DNA, which was then amplified by PCR. The polymorphism of 27 bp repeat sequence in intron 4 and G894T polymorphism in exon 7 of eNOS gene was detected. Results: The b/b, b/a and a/a genotype frequency of intron 4 was 77.7%, 19.2% and 3.1% in ANFH group, respectively, and that in the control group was 58.0%, 32.8% and 9.2%, respectively. The b allele frequency in ANFH group was obviously higher than that in the control (P<0.0001). The frequency of 894 G/G wild type, G/T heterozygote and T/T homozygote in eNOS exon 7 was analyzed by PCR-RLFP: 65.4%, 26.5% and 8.1% in ANFH group, and 46.2%, 37.8% and 16% in normal control, respectively. The frequency of TT genotype in ANFH was obviously higher than that in the control group (P<0.001). Conclusion: Polymorphism of eNOS was correlated with ANFH.
Keywords: Polymorphism/genetics, endothelial cells/enzymology, nitric oxide synthase (NOS)/genetics, ischemia, necrosis of femoral head/genetics
Introduction
Avascular necrosis of femoral head (ANFH) is a common joint disease in China which affects millions of people. Pathogenic factors of ANFH include genetics, injuries, hormone abuse, alcohol abuse and coagulation factors [1-3]. These factors can lead to reduced blood supply to femoral head, osteonecrosis and finally the collapse of bone cortex and osteochondral destruction. The future direction of AHFH treatment is early discovery and early prevention, so as to reverse or slow down the progression.
Endothelial nitric oxide synthase (eNOS) is responsible for the production of nitric oxide (NO) and reduces vascular tension and thrombosis risk. Recent studies have shown that two polymorphic loci of eNOS gene, namely, 27 bp repeat sequence in intron 4 and G894y polymorphism in exon 7, are related to coronary heart disease, hypertension and nephropathy [4-6]. Though the connections between eNOS polymorphism and vascular diseases are established, very few reports are published concerning the relationship between eNOS gene and ANFH [7]. We observed the effects of 27 bp repeat sequence in intron 4 and G894y polymorphism in exon 7 of eNOS gene on the incidence of ANFH. The polymorphism of the two loci can be used for the screening of high-risk groups.
Materials and methods
General data
ANFH patients treated at Department of Orthopedics of our hospital from January 2008 to December 2014 were selected. Excluding those with a history of hip joint injuries, 260 cases were finally recruited (157 males and 103 females, aged 55.4±10.2 years old). By pathogenic factors, the cases were divided into idiopathic ANFH, hormone-induced ANFH and alcohol-induced ANFH. All cases were diagnosed and subject to staging according to Ficat standard and relevant staging criteria. For control group, 262 healthy people receiving physical examination at our hospital were included (161 males and 101 females, aged 55.9±10.5 years old). All cases signed informed consent. The tube containing EDTA as anticoagulant was used to collect 5 mL of venous blood from each case. Peripheral blood monocular cells (PBMC) were isolated by Ficoll method and cryopreserved.
Equipment and reagents
Milli-Q Ultrapure Water Systems (Millipore Corporation, USA), BD202 Balance (Metfler-Toledo International Inc., Germany), UV spectrophotometer (Eppendorf, Germany), ABI PRISM 310 Genetic Analyzer (PE Corporation, USA), 1640 culture medium (GIBCO, USA); DNeasy Tissue Kit (QIAGEN, Germany), RNase (Roche, Switzerland), Gel Extraction Kit (MACHER-EY-NAGEL, USA), BigDye Sequencing Kit (Applied Biosystems, USA).
Experimental methods
DNA extraction and purification detection
Frozen PBMC specimens were thawed and used for genome DNA extraction with DNeasy Tissue Kit (QIAGEN, Germany). Cell lysis buffer was added and cell membrane was disrupted by oscillation. For each specimen 100 μL of RNaseA was added. The specimen was passed through CB6 column and TB buffer was used for elution. Genome DAN extraction was carried out according to the kit instruction. The recovered DNA fragments were detected for concentration and purity by 0.8% agarose gel electrophoresis and ultraviolet spectroscopy. Images were taken using FR-200 UV-Vis detector for quantification by referring to DNA markers.
Synthesis of specific primers and PCR amplification
Genbank was searched for base sequences of 27 bp repeat sequence in intron 4 and G894T polymorphism in exon 7 of eNOS gene. Primers were designed using Primer 5.0 (see Table 1). Conditions of PCR amplification were as follows: denaturation at 95°C for 15 min, at 95°C for 30 s, annealing at 55°C for 30 s, extension at 72°C for 1 min, 33 cycles, final extension at 72°C for 5 min. The PCR products were separated by agarose gel electrophoresis and the fragments were recovered using Gel Extraction Kit (MACHEREY-NAGEL).
Table 1.
The primer sequences of the two loci
| Locus | Sense primer | Antisense primer |
|---|---|---|
| 27 bp repeat polymorphism | 5’AGGCCCTATGGTAGTGCCTT3’ | 5’TCTCTTAGTGCTGTGGTCAC3’ |
| G894T polymorphism | 5’AAGGCAGGAGACAGTGGAGGT3’ | 5’CCCAGTCAATCCCTTTGGTGCTCA3’ |
Agarose gel electrophoresis of DNA
The 1.5% agarose gel was filled into the tank and solidified, and the specimen was loaded. Electrophoresis was carried out for 2 h at the voltage of 3-5 V/cm, and then the gel was removed and stained with ethidium bromide. Electrophoresis patterns were obtained at the wave length of 254 nm.
Restriction enzyme digestion for exon 7
The system of restriction enzyme digestion consisted of the followings: 1 μL Ban II, 2 μL 10 X Buffer, DNA≤1 μg, and sterile water up to 20 μL. The reaction temperature was 37°C. After digestion at 37°C for 1 h in the above 20 μL reaction system, 10 μL of product of digestion was collected and mixed with 6 X loading buffer. Agarose gel electrophoresis was carried out at the 100 V for 30-60 min.
Sequencing of intron 4
PCR products were purified by using sodium acetate/ethanol method and pretreated before electrophoresis. Capillary tube was mounted according to the instruction and the position of the tube was calibrated. Gel filling was performed manually and the sequential files were established. Then the gel was automatically filled into the capillary tube. After pre-electrophoresis at 1.2 kV for 5 min, sample loading was performed automatically according to the programmed sequence. This was followed by pre-electrophoresis (1.2 kV, 20 min) and electrophoresis at 7.5 kV for 2 h. When the electrophoresis was over, the equipment would be cleaned automatically. The next round of gel filling, pre-electrophoresis and electrophoresis began for the new specimen. The total electrophoresis time for each specimen was 2.5 h. The color sequencing chromatograms were printed automatically.
Statistical processes
All statistical analyses were performed using SPSS 18.0. Data were compared with X2 test or t test, and P<0.05 was considered as statistically significant.
Results
Clinical data of subjects
The course of disease in 260 ANFH cases was 3 months to 23 years, with an average of 110.2±60.1 months. Staging results showed that 38 cases belonged to stage II, 75 to stage III (including transitional cases) and 147 to stage IV. According to etiological classification, 65, 132 and 63 cases were alcohol-induced ANFH, idiopathic ANFH and hormone-induced ANFH, respectively. The characteristics of the patients and the control were shown in the Table 2.
Table 2.
Characteristics of the participants
| Groups | N | Age (years) | Gender (M/F) | BMI Kg/m2 | GLU (mmol/L) | TG (mmol/L) | TC (mmol/L) | HDL-C (mmol/L) | LDL-C (mmol/L) |
|---|---|---|---|---|---|---|---|---|---|
| ANFH group | 260 | 55.4±10.2 | 157/103 | 24.1±3.3 | 5.9±1.6 | 2.1±1.3 | 5.5±2.7 | 1.7±0.9 | 2.7±1.5 |
| Control group | 262 | 55.9±10.5 | 161/101 | 24.4±3.4 | 5.6±1.3 | 1.7±1.4 | 4.9±2.6 | 1.6±0.8 | 2.6±1.7 |
| P | 0.432 | 0.417 | 0.112 | 0.652 | 0.079 | 0.088 | 0.184 | 0.559 |
Note: BMI=Body mass index; GLU=Glucose; TC=Total cholesterol; LDL-C=Low-density lipoprotein-cholesterol; LDL-C=High-density lipoprotein-cholesterol; TG=Triglycerides.
Detection of G894T polymorphism in exon 7
The amplified products were hydrolyzed by specific endonucleases. Wild-type DNA was hydrolyzed by BanII into two fragments of unequal length, corresponding to 163 bp and 85 bp bands on the electrophoretograms, respectively. However, mutant DNA could not be hydrolyzed with a length of 248 bp, corresponding to the third band on the electrophoretogram (Figure 1).
Figure 1.
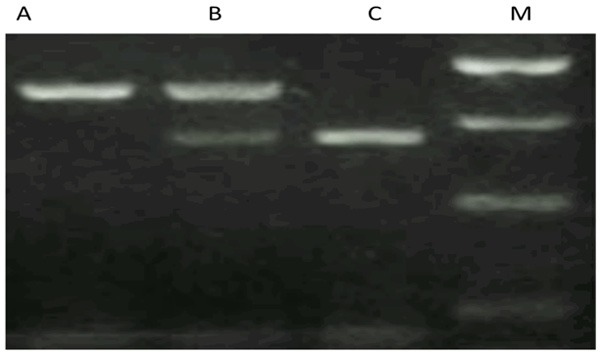
Genotyping results of G894T polymorphism in exon 7. A: TT genotype; B: GT genotype; C: GG genotype; M: DNA marker.
Polymorphism detection of 27 bp repeat sequence in intron 4 of eNOS gene
Sequencing of Intron 4 of eNOS gene polymorphism indicated that three polymorphisms, which were b/b, b/a and a/a (Figure 2).
Figure 2.
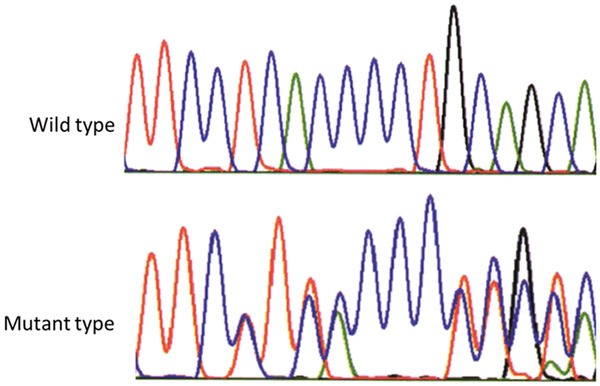
The wild type and mutant type sequences 27 bp repeat polymorphism of NOS gene.
Data analysis
Distribution of eNOS alleles in normal people and ANFH cases
The frequencies of intron 4 b allele and 894T allele in exon 7 in ANFH group were obviously higher than those of the control (P<0.001, Table 3).
Table 3.
Distribution of genotype and alleles between two groups
| SNP | Groups | N | Genotype | P | Allele | P | |||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||
| a/a | a/b | b/b | a | b | |||||
| 27 bp repeat polymorphism | ANFH group | 260 | 8 (3.1) | 50 (19.2) | 202 (77.7) | <0.001 | 66 (12.7) | 454 (87.3) | <0.001 |
| Control group | 262 | 24 (9.2) | 86 (32.8) | 152 (58.0) | 134 (25.7) | 390 (74.3) | |||
| TT | GT | GG | G | T | |||||
| G894T | ANFH group | 260 | 21 (8.1) | 69 (26.5) | 170 (65.4) | <0.001 | 111 (21.3) | 409 (78.7) | <0.001 |
| Control group | 262 | 42 (16.0) | 99 (37.8) | 121 (46.2) | 183 (34.9) | 341 (65.1) | |||
The frequency of eNOS intron 4 b/a genotype in ANFH group was significantly higher than that of the normal control (P<0.0001). The frequencies of G894T polymorphism in exon 7 and G/T genotype in ANFH group were considerably higher than those of the control (P<0.001, Table 3).
Discussion
In the present study, we found genetic polymorphisms of eNOS were associated with ANFA in a Chinese population. The risk factors of ANFH include use of steroid hormones, alcohol abuse, trauma and genetics [2,3,8]. The cases of ANFH may present no typical symptoms during early onset and there is a lack of practical screening tool. Along with progress of medical sciences, the correlation between ANFH and gene polymorphism has drawn increasing attention. New molecular methods are proposed for treating ANFH. We aimed to study the correlation between the polymorphism of eNOS gene and the risk of ANFH based on the molecular mechanism of ANFH, so as to offer new technique for screening, early diagnosis and intervention.
NO produced by eNOS has a wide range of bioactivity, including anti-oxidative, anti-inflammatory, anticoagulation, antiplatelet aggregation and adhesion [9-12]. eNOS in bones is involved in the regulation of bone turnover. The normal functioning of some hormones such as IGF and estrogen also relies on eNOS [13]. Directly acting on platelets, NO can inhibit platelet aggregation and adhesion and reduce platelet thrombosis formation. NO can facilitate wound healing by promoting angiogenesis [14]. Given these factors, the author believes that eNOS is involved in ANFH pathogenesis. Two pathogenic mutation loci have been found in eNOS gene. One is G894T polymorphism in exon 7 of eNOS gene, leading to the change from glutamic acid to aspartic acid at site 298 of peptide. The other is the mutation of five 27 bp repeat sequences (b allele, wild type) in intron 4 into four 27 bp repeat sequences and one inverted repeat sequence (a allele, mutant type). It is generally believed that intronic mutation affects mRNA transcription, while exonic mutation affects the function and activity of enzymes [15]. Recently, Tesauro et al. [16] showed that eNOS mutant gene was less stable than that by wild-type gene. Although mutation had no impact on the substrate binding ability and activity of enzyme, the peptide encoded by mutant eNOS gene was more easily hydrolyzed than that by wild-type eNOS gene. As a result, eNOS content was reduced and NO production declined accordingly. Besides, the plasma level of NO in individual with eNOS intron 4a mutation was obviously lower than that with wild-type eNOS gene [17]. That is to say, the mutation of two loci reduced NO content.
The correlations of polymorphism of eNOS with coronary heart disease, hypertension and strokes have been established in many studies [18-21]. We observed that a significantly higher number of ANFH cases carried mutant intron 4 of eNOS gene than normal controls, and this difference was especially prominent in idiopathic ANFH. Since the mutation of intron 4 can reduce eNOS synthesis, it is inferred that intron 4a mutation is one risk factor of ANFH. NO produced by eNOS has a protective effect on femoral bones and is therefore considered as a beneficial factor.
Alcohol and steroid hormone abuse is the non-traumatic risk factor of ANFH. Some reports showed that glucocorticoids inhibit eNOS gene expression, but we did not find obvious differences in allele frequency between hormone-induced ANFH and normal controls. The possible reason may be the limited sample size. However, few data are available with respect to the influence of alcohol on eNOS gene expression, so no conclusive findings have been reached. We did not find obvious differences in allele frequency between alcohol-induced ANFH and normal controls, which may be also due to the limited sample size.
Frequencies of intron 4 b/a genotype and G894T polymorphism in exon 7 were calculated and compared with those of normal controls. PBMC isolation, cell whole-genome extraction, specific PCR amplification, determination of nucleotide sequencing of target genes and polymorphism analysis of DNA fragments by restriction enzyme digestion were performed. The results showed that ANFH group and normal control group differed in the polymorphism of eNOS gene. It was discovered that intron 4 b/a genotype of eNOS gene among Chinese population caused the increase of ANFH risk. G894T polymorphism in exon 7 also led to an obvious rise of ANFH incidence. In order to confirm the correlation between the polymorphism of eNOS gene and idiopathic ANFH, the sample size should be enlarged. Therefore, polymorphism of eNOS gene provides a candidate target for the screening of high-risk population and early intervention of ANFH.
Disclosure of conflict of interest
None.
References
- 1.Peng KT, Huang KC, Huang TW, Lee YS, Hsu WH, Hsu RW, Ueng SW, Lee MS. Single nucleotide polymorphisms other than factor V Leiden are associated with coagulopathy and osteonecrosis of the femoral head in Chinese patients. PLoS One. 2014;9:e104461. doi: 10.1371/journal.pone.0104461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akodu SO, Diaku-Akinwumi IN, Kehinde OA, Njokanma OF. Evaluation of arm span and sitting height as proxy for height in children with sickle cell anemia in Lagos, Nigeria. J Am Coll Nutr. 2014;33:437–41. doi: 10.1080/07315724.2013.875356. [DOI] [PubMed] [Google Scholar]
- 3.Rai P, Takwale V. A Debilitating Orthopaedic Complication following Corticosteroid Therapy for Polymyalgia Rheumatica. Case Rep Rheumatol. 2014;2014:515361. doi: 10.1155/2014/515361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tantawy AA, Adly AA, Ismail EA, Aly SH. Endothelial nitric oxide synthase gene intron 4 variable number tandem repeat polymorphism in β-thalassemia major: relation to cardiovascular complications. Blood Coagul Fibrinolysis. 2015;26:419–25. doi: 10.1097/MBC.0000000000000277. [DOI] [PubMed] [Google Scholar]
- 5.Kocyigit I, Taheri S, Sener EF, Unal A, Eroglu E, Öztürk F, Korkmaz K, Zararsiz G, Imamoglu H, Sipahioglu MH, Tokgoz B, Oymak O. Endothelial nitric oxide synthase gene expression is associated with hypertension in autosomal dominant polycystic kidney disease. Cardiorenal Med. 2014;4:269–79. doi: 10.1159/000369105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shankarishan P, Borah PK, Ahmed G, Mahanta J. Endothelial nitric oxide synthase gene polymorphisms and the risk of hypertension in an Indian population. Biomed Res Int. 2014;2014:793040. doi: 10.1155/2014/793040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zheng L, Wang W, Ni J, Li Z, Xiao T. The association of eNOS gene polymorphism with avascular necrosis of femoral head. PLoS One. 2014;9:e87583. doi: 10.1371/journal.pone.0087583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Felten R, Messer L, Moreau P, Goussot R, Mahé A. Osteonecrosis of the femoral head linked to topical steroids for skin bleaching: a case report. Ann Intern Med. 2014;161:763–4. doi: 10.7326/L14-5026. [DOI] [PubMed] [Google Scholar]
- 9.Frendl CM, Tucker SM, Khan NA, Esch MB, Kanduru S, Cao TM, García AJ, King MR, Butcher JT. Endothelial retention and phenotype on carbonized cardiovascular implant surfaces. Biomaterials. 2014;35:7714–23. doi: 10.1016/j.biomaterials.2014.05.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shen GL, Lv H, Bi HY, Zhang W, Yao S, Yuan Y. Reduction expression of thrombomodulin and endothelial cell nitric oxide synthase in dermatomyositis. Neuropathology. 2007;27:309–13. doi: 10.1111/j.1440-1789.2007.00779.x. [DOI] [PubMed] [Google Scholar]
- 11.Shen GL, Lü H, Bi HY, Zhang W, Yao S, Tu P, Yuan Y. Immunopathological changes of micro-vessels in dermatomyositis. Zhonghua Yi Xue Za Zhi. 2006;86:1912–5. [PubMed] [Google Scholar]
- 12.Uchiyama T, Atsuta H, Utsugi T, Oguri M, Hasegawa A, Nakamura T, Nakai A, Nakata M, Maruyama I, Tomura H, Okajima F, Tomono S, Kawazu S, Nagai R, Kurabayashi M. HSF1 and constitutively active HSF1 improve vascular endothelial function (heat shock proteins improve vascular endothelial function) Atherosclerosis. 2007;190:321–9. doi: 10.1016/j.atherosclerosis.2006.03.037. [DOI] [PubMed] [Google Scholar]
- 13.Duckles SP, Miller VM. Hormonal modulation of endothelial NO production. Pflugers Arch. 2010;459:841–51. doi: 10.1007/s00424-010-0797-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.López-Farré AJ, Modrego J, Azcona L, Guerra R, Segura A, Rodríguez P, Zamorano-León JJ, Lahera V, Macaya C. Nitric oxide from mononuclear cells may be involved in platelet responsiveness to aspirin. Eur J Clin Invest. 2014;44:463–9. doi: 10.1111/eci.12252. [DOI] [PubMed] [Google Scholar]
- 15.Korir PK, Roberts L, Ramesar R, Seoighe C. A mutation in a splicing factor that causes retinitis pigmentosa has a transcriptome-wide effect on mRNA splicing. BMC Res Notes. 2014;7:401. doi: 10.1186/1756-0500-7-401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tesauro M, Thompson WC, Rogliani P, Qi L, Chaudhary PP, Moss J. Intracellular processing of endothelial nitric oxide synthase isoforms associated with differences in severity of cardiopulmonary diseases: cleavage of proteins with aspartate vs. glutamate at position 298. Proc Natl Acad Sci U S A. 2000;97:2832–5. doi: 10.1073/pnas.97.6.2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boonla O, Kukongviriyapan U, Pakdeechote P, Kukongviriyapan V, Pannangpetch P, Prachaney P, Greenwald SE. Curcumin improves endothelial dysfunction and vascular remodeling in 2K-1C hypertensive rats by raising nitric oxide availability and reducing oxidative stress. Nitric Oxide. 2014;42:44–53. doi: 10.1016/j.niox.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 18.Gaunt TR, Davey Smith G. eNOS and coronary artery disease: publication bias and the eclipse of hypothesis-driven meta-analysis in genetic association studies. Gene. 2015;556:257–8. doi: 10.1016/j.gene.2014.11.052. [DOI] [PubMed] [Google Scholar]
- 19.Vecoli C. Endothelial nitric oxide synthase gene polymorphisms in cardiovascular disease. Vitam Horm. 2014;96:387–406. doi: 10.1016/B978-0-12-800254-4.00015-5. [DOI] [PubMed] [Google Scholar]
- 20.Matyar S, Acartürk E, Attila G, Ünal I, Soyer L, Akpınar O. Gene-gene interaction of ACE I/D, endothelial nitric oxide synthase 4 a/b and ApoE does not affect coronary artery disease severity. Adv Clin Exp Med. 2014;23:215–23. doi: 10.17219/acem/37065. [DOI] [PubMed] [Google Scholar]
- 21.Fang C, Ren X, Zhou H, Gong ZC, Shen L, Bai J, Yin JY, Qu J, Li XP, Zhou HH, Liu ZQ. Effects of eNOS rs1799983 and ACE rs4646994 polymorphisms on the therapeutic efficacy of salvianolate injection in Chinese patients with coronary heart disease. Clin Exp Pharmacol Physiol. 2014;41:558–64. doi: 10.1111/1440-1681.12257. [DOI] [PubMed] [Google Scholar]


