Abstract
Objective: This study aims to elucidate the roles of PD-1, Tim-3 and CTLA-4 in sepsis. Methods: Sepsis patients (n = 182) were selected as sepsis group and divided into three subgroups: mild sepsis group, severe sepsis group and septic shock group; 185 healthy volunteers were enrolled as control group. Flow cytometry and blood routine examination were performed for T lymphocytes and surface co-stimulatory molecules expressions. Pearson correlation test was applied for the correlation of co-stimulatory molecules expressions on T lymphocytes with critical illness in sepsis. Logistic regression analysis was conducted for risk factors in sepsis. Results: Heart rate and WBC in subgroups were higher than control group (P < 0.05). The differences in APACHE II, SAP II and SOFA score among subgroups were statistically significant (P < 0.05). Compared with control group, lymphocyte ratio and percentage of CD4+ T cells reduced in subgroups (P < 0.05). The differences in expression levels of CD4+PD-1+, CD8+PD-1+, and CD8+CTLA-4+ showed statistical significances (P < 0.05). Apparently, expression levels of CD4+TIM-3+, CD8+TIM-3+, CD4+PD-1+, CD8+PD-1+, and CD4+CTLA-4+ were positively correlated with APACHE II score (all P < 0.05). Logistic regression analysis showed that heart rate and expression level of CD4+PD-1+ might be risk factors while the percentage of CD4+ T cells might be a protective factor for sepsis (P < 0.05). Conclusion: PD-1 aggravates immune responses consistent with promotion of T cell exhaustion in sepsis. Expression level of CD4+PD-1+ and heart rate are potential risk factors while percentage of CD4+ T cells is a possible protective factor for sepsis.
Keywords: Sepsis, PD-1, Tim-3, CTLA-4, Regulatory T cell, APACHE II score, SAP II score, SOFA score
Introduction
Sepsis, a whole-body inflammation, is the first leading cause of mortality in most intensive care units (ICU) [1]. In the developed world, approximately 0.2-3 per 1000 people are diagnosed with sepsis annually and about a million cases of sepsis per year in the United States [2,3]. Common signs and symptoms of sepsis involve fever, increased heart rate, increased respiratory rate, confusion, a cough with pneumonia or painful urination, and a kidney infection [4]. Severe sepsis causes poor organ function or insufficient blood flow that may be evidenced by low blood pressure, high blood lactate, or low urine output; septic shock is sepsis-induced low blood pressure that does not be improved after given reasonable amounts of intravenous fluids [3]. The cause of sepsis is an immune response triggered by an infection, most commonly by bacteria, common locations for which include lungs, blood, brain, urinary tract, skin, and abdominal organs [5]. Environmental risk factors include young or old age, a weakened immune system from conditions, such as cancer or diabetes, and major trauma or burns [6]. On the other hand, numerous researches revealed that programmed death 1 (PD-1) and cytotoxic T lymphocyte-associated antigen-4 (CTLA-4) might be associated with sepsis by regulating the regulatory T cells [1,7,8].
PD-1, as a member of the B7-CD28 superfamily, functions to be a co-inhibitory receptor, the expression of which can be induced primarily on the cell surface of activated CD4 and CD8 T cells [9]. PD-1 and its two main ligands including PD-L1 (B7-H1) and PD-L2 (B7-DC) are able to inhibit the setting of persistent antigenic stimulation through the regulation of the balance of activation, tolerance, and immunopathology in T cells [10]. Studies recently propose a critical role of PD-1/PD-L1 pathway in the interaction between host and pathogenic microbes, which evolved to resist immune responses, such as, allergy, immune privilege, autoimmunity, tumor immunity, ischemia/reperfusion injury, and transplantation immunity [11,12]. T cell immunoglobulin-and mucin-domain-containing molecule 3 (Tim-3) is a negative regulator of IFN-γ-secreting CD4+ T helper 1 and CD8+ T cytotoxic 1 cells identified nearly 10 years ago [13]. Engagement of Tim-3 by its ligand (galectin-9) has a great effect on inducing the tolerance in T cell in both mouse models and human experiments [14]. Tim-3 has been highlighted recently as a crucial player in the CD8+ T cell exhaustion, which occurs in chronic immune conditions, for instance, chronic viral infection [15,16]. CTLA-4 is a protein which is expressed on the surface of activated T cells subsequent to the engagement of T-cell receptor and CD28 [17]. By interacting with two ligands, CD80 (B7-1) and CD86 (B7-2), on antigen-presenting cells, CTLA-4 can potently inhibit the T cell immune responses [18]. Multiple molecular mechanisms are suggested for the way in which CTLA-4 exerts an inhibitory effect on T-cell activation, relating to successful competition with CD28 for ligand binding or ligand removal by transendocytosis [19,20]. It has been documented that immuno-adjuvant therapy with anti-PD-1, anti-CTLA-4 and anti-Tim-3 antibodies is able to reverse sepsis-induced immunosuppression and improve survival in sepsis patients [1,21].
In our present study, the purpose is to elucidate the roles of PD-1, Tim-3, and CTLA-4 in sepsis. In addition, our study aimed to explore the potential mechanisms underlying the putative beneficial effects of PD-L, Tim-3 and CTLA-4 antagonism in patients with sepsis.
Materials and methods
Ethnic statement
This study was approved by the Ethical Committee of the First Affiliated Hospital of Dalian Medical University. All study participants or their family members provided written informed consent.
Study subjects
All subjects were divided into sepsis group and control group. Between September 2013 and December 2014, 182 sepsis patients (male, 105; female, 77; age range, 19-88 years; mean age, 53.51 ± 14.51 years) in the ICU were selected from the First Affiliated Hospital of Dalian Medical University as the sepsis group. Sepsis was diagnosed based on the diagnostic criteria proposed by 2001 Society of Critical Care Medicine (SCCM)/European Society of Intensive Care Medicine (ESICM)/American College of Chest Physicians (ACCP)/American Thoracic Society (ATS)/Surgical Infection Society (SIS) International Sepsis Definitions Conference [22]. According to these diagnosis criteria, all patients with sepsis were divided into three subgroups: mild sepsis group (n = 54; male, 32; female, 22; mean age, 54.2 ± 14.3 years), severe sepsis group (n = 46; male, 29; female, 17; mean age, 58.5 ± 13.2 years), and septic shock group (n = 82; male, 50; female, 32; mean age, 56.5 ± 14.8 years). During the corresponding period, another 185 healthy volunteers (male, 111; female, 74; age range, 26-79 years; mean age, 56.2 ± 12.3 years), undergoing a physical examination in the outpatient department, were enrolled as the control group. No statistical difference in age was detected between the four groups (all P > 0.05).
Inclusion criteria of sepsis patients were: (1) body temperature > 38°C or < 36°C; (2) heart rate > 90 times/min; (3) respiratory rate > 20 times/min; (4) peripheral blood white blood cell count (WBC) > 12 × 109 [23]. Sepsis patients met any above 3 of 4 inclusion criteria were enrolled in the sepsis group. Exclusion criteria were: (1) patients with severe trauma, immunodeficiency virus (HIV) or hematologic diseases; (2) patients with tumor or chronic diarrhea; (3) patients received an organ transplant; (4) patients with chronic hepatic and renal insufficiency; (5) patients during pregnancy or lactation.
Flow cytometry for T lymphocytes and surface co-stimulatory molecules expressions
Briefly, 2-3 mL peripheral venous blood samples were collected and anticoagulated with ethylenediaminetetraacetic acid (EDTA; PERLONG, Beijing, China). After mixed well, 100 μL of each whole blood sample was sucked into 5 different test tubes. Monoclonal antibodies (20 μL) against CD4 and CD8 were added into the first test tube, monoclonal antibodies (20 μL) against CD4, CD8 and CTLA-4 into the second test tube, monoclonal antibodies (20 μL) against CD4, CD8 and PD-1 into the third test tube, monoclonal antibodies (20 μL) against CD4, CD8 and Tim-3 into the fourth test tube, IgG-FITC, IgG-PE and IgG-APC into the fifth test tube as homotype control. After being mixed well, all test tubes were cultured under dark for 30 min at room temperature, and then added with 0.5 mL fresh hemolytic agent (hemolysin: distilled water = 1:9; PERLONG, Beijing, China), and cultured under dark for 12 min after being mixed well. Subsequently, all test tubes were centrifuged at 300 × g for 5 min, and washed with phosphate buffer solution (PBS) 3 times. Supernatant was discarded, PBS (0.3 mL) was added into each test tube and stored at 4°C after being mixed well, for detection within 24 h. Lymphocyte counts, lymphocyte ratio, percentage of CD4+ cells, percentage of CD8+ cells, and the expression rates of CTLA-4, PD-1 and Tim-3 on CD4+ and CD8+ cells were determined, respectively.
General clinical parameters detection and blood routine examination
Age and gender of all study subjects were recorded. Vital signs, including body temperature, heart rate, respiratory rate and WBC of all patients were monitored. Automatic blood cell analyzer (Beckman Coulter) was used to detectthe absolute value of lymphocyle (× 109/L). The expression of CTLA-4, PD-1 and Tim-3 (× 106/L) = the absolute value of lymphocyte × the expression rate of CTLA-4, PD-1 and Tim-3. Acute physiology and chronic health evaluation (APACHE) II score, simplified acute physiology (SAPA) II score and sequential organ failure assessment (SOFA) score of all patients were calculated [24].
Statistical analysis
The statistic software SPSS19.0 was used to carry out all the statistical analysis. The measurement data were expressed by mean ± standard deviation (SD). The single factor analysis of variance was used for the comparisons among multiple groups, and the least significant difference t (LSD-t) test was used for the comparisons between groups. Comparisons of enumeration data were carried out utilizing the chi-square test. Correlation analysis was conducted by using the Pearson correlation test. All P values were bilateral and a value of P < 0.05 was considered statistically significant.
Results
Clinical characteristics
There was no statistically significant difference in age or gender between the control group and those three subgroups (all P > 0.05). In the subgroups, both heart rate and WBC were higher than the control group (all P < 0.05). The WBC in the septic shock group and the severe sepsis group were also higher than the mild sepsis group (both P < 0.05). The respiratory rate of the severe sepsis group and the septic shock group were higher than that of the control group (both P < 0.05); in addition, the respiratory rate of the septic shock group was also higher than that of the mild sepsis group (P < 0.05). The differences in APACHE II score, SAP II score and SOFA score between the subgroups were statistically significant (all P < 0.05). APACHE II score, SAP II score and SOFA score in the septic shock group were higher than those in the mild septic group; additionally, APACHE II score and SOFA score in the septic shock group were higher than those in the severe septic group (all P < 0.05) (Table 1).
Table 1.
Clinical characteristics of patients with mild sepsis, severe sepsis and septic shock and healthy controls
| Groups | Control group (n = 185) | Mild sepsis group (n = 54) | Severe sepsis group (n = 46) | Septic shock group (n = 82) |
|---|---|---|---|---|
| Age (year) | 56.2 ± 12.3 | 54.2 ± 14.3 | 58.5 ± 13.2 | 56.5 ± 14.8 |
| Male/Female (case) | 111/74 | 32/22 | 29/17 | 50/32 |
| Body temperature (°C) | 36.9 ± 3.8 | 38.7 ± 3.9 | 38.8 ± 4.6 | 39.5 ± 4.4a |
| Heart rate (times/min) | 95.27 ± 9.36 | 102.95 ± 8.07a | 104.71 ± 9.07a | 105.11 ± 9.07a |
| Respiratory rate | 18.49 ± 5.30 | 20.08 ± 3.89 | 21.27 ± 3.43a | 22.21 ± 4.01a,b |
| WBC (× 109/L) | 9.45 ± 3.23 | 11.67 ± 4.05a | 14.62 ± 3.01a,b | 14.98 ± 5.12a,b |
| APACHE II score | - | 16.26 ± 4.32 | 22.34 ± 5.12b | 25.26 ± 6.28b,c |
| SAPS II score | - | 23.51 ± 11.21 | 39.26 ± 9.30b | 42.20 ± 10.95b |
| SOFA score | - | 7.25 ± 1.27 | 8.12 ± 1.26b | 10.59 ± 1.53b,c |
Note: WBC, white blood cell; APACHE, acute physiology and chronic health evaluation; SAPS, simplified acute physiology; SOFA, sequential organ failure assessment;
compared with the control group, P < 0.05;
compared with themild sepsis group, P < 0.05;
compared with the severe sepsis group, P < 0.05.
Comparisons of peripheral blood T lymphocyte subsets
Compared with the control group, both lymphocyte ratio and percentage of CD4+ T cells in peripheral blood reduced in all subgroups (all P < 0.05). Lymphocyte ratio and percentage of CD4+ T cells in peripheral blood of the severe sepsis group and septic shock group were also lower in comparison with those of the mild sepsis group (all P < 0.05). The percentage of CD4+ T cells in the septic shock group was lower than the severe sepsis group (P < 0.05). Lymphocyte count in the severe sepsis group and the septic shock group were lower than the control group (both P < 0.05). Compared with the mild sepsis group, lymphocyte count in the septic shock group was lower (P < 0.05) (Table 2).
Table 2.
Comparisons of lymphocytes between the sepsis groups (mild sepsis group, severe sepsis group and septic shock group) and the control group (x̅ ± s)
| Groups | Control group (n = 185) | Mild sepsis group (n = 54) | Severe sepsis group (n = 46) | Septic shock group (n = 82) |
|---|---|---|---|---|
| Lymphocyte ratio (%) | 29.67 ± 6.87 | 16.18 ± 5.16a | 15.83 ± 5.36a | 16.54 ± 5.35a,b |
| Lymphocyte count (%) | 1.64 ± 0.58 | 1.54 ± 0.46 | 1.36 ± 0.42a | 1.21 ± 0.48a,b |
| CD4+ T (%) | 59.27 ± 7.23 | 34.99 ± 8.68a | 34.71 ± 8.80a,b | 35.98 ± 9.69a,b,c |
| CD8+ T (%) | 33.14 ± 7.63 | 32.31 ± 8.07 | 31.24 ± 7.08 | 30.85 ± 8.06 |
compared with the control group, P < 0.05;
compared with the mild sepsis group, P < 0.05;
compared with the severe sepsis group, P < 0.05.
Comparisons of co-stimulatory molecules on T lymphocytes
As seen in Table 3, there were no statistically significant differences in CD4+TIM-3+, CD8+TIM-3+ and CD4+CTLA-4+ expression levels between all groups (all P > 0.05); the differences in the expression levels of other co-stimulatory molecules (CD4+PD-1+, CD8+PD-1+, CD8+CTLA-4+) between groups did show statistical significance (all P < 0.05). Compared with the control group, the expression levels of CD4+CTLA-4+, CD4+PD-1+ and CD8+PD-1+ in all subgroups were higher (all P < 0.05) (as seen in Table 3 and Figure 1).
Table 3.
Comparisons of co-stimulatory molecules on T lymphocytes between the sepsis groups (mild sepsis group, severe sepsis group and septic shock group) and the control group (x̅ ± s, × 106/L)
| Groups | Control group (n = 185) | Mild sepsis group (n = 54) | Severe sepsis group (n = 46) | Septic shock group (n = 82) |
|---|---|---|---|---|
| CD4+TIM-3+ | 62.3 ± 5.7 | 61.0 ± 6.2 | 60.4 ± 6.4 | 62.5 ± 6.0 |
| CD8+TIM-3+ | 4.3 ± 1.2 | 4.6 ± 1.3 | 4.6 ± 1.7 | 4.7 ± 1.6 |
| CD4+PD-1+ | 21.8 ± 3.0 | 23.9 ± 2.1a | 24.3 ± 3.6a | 24.6 ± 3.3a |
| CD8+PD-1+ | 3.1 ± 0.7 | 3.6 ± 0.7a | 3.8 ± 1.1a | 3.9 ± 1.5a |
| CD4+CTLA-4+ | 116.8 ± 14.1 | 117.8 ± 16.0 | 116.6 ± 12.8 | 120.9 ± 12.1 |
| CD8+CTLA-4+ | 37.6 ± 6.9 | 40.7 ± 7.8a | 42.4 ± 5.2a | 43.7 ± 7.1a |
compared with the control group, P < 0.05.
Figure 1.
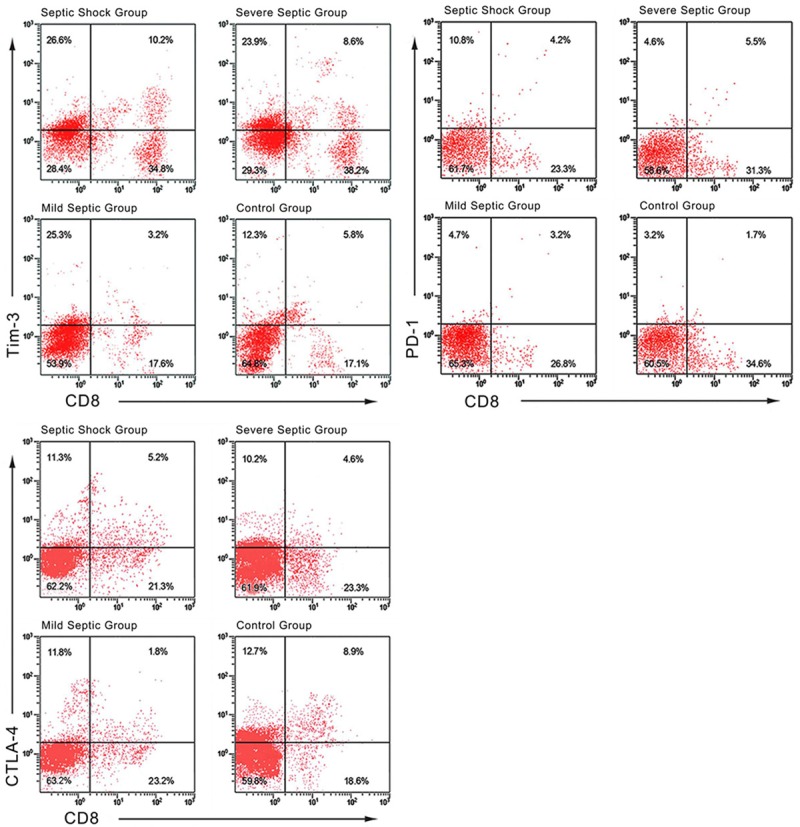
Flow cytometry analysis of the expression levels of co-stimulatory molecules on T lymphocytes in sepsis patients and healthy controls.
Correlations ofco-stimulatory molecules expresionson T lymphocytes withthe critical illnessin sepsis
Correlation analysis showed that no correlation between the expression levels of co-stimulatory molecules on T lymphocytes and SAPS II score was found. Apparently, the expression levels of CD4+TIM-3+, CD8+TIM-3+, CD4+PD-1+, CD8+PD-1+, and CD4+CTLA-4+ were positively correlated with APACHE II score (all P < 0.05). In addition, certain positive correlations were detected to exist between the expression levels of CD4+TIM-3+, CD8+TIM-3+, CD4+PD-1+, CD8+PD-1+, and CD4+CTLA-4+ and SOFA score (all P < 0.05) (Table 4).
Table 4.
Correlation analysis for critical illness score and expression levels of co-stimulatory molecules on T lymphocytes
| Groups | APACHE II score | SAPS II score | SOFA score |
|---|---|---|---|
| CD4+TIM-3+ | 0.728* | 0.017 | 0.651* |
| CD8+TIM-3+ | 0.827* | 0.003 | 0.679* |
| CD4+PD-1+ | 0.861* | 0.059 | 0.731* |
| CD8+PD-1+ | 0.867* | 0.017 | 0.732* |
| CD4+CTLA-4+ | 0.728* | 0.067 | 0.651* |
| CD8+CTLA-4+ | 0.103 | 0.155* | 0.133 |
Note: figures in the table were correlation coefficients;
P < 0.05;
APACHE, acute physiology and chronic health evaluation; SAPS, simplified acute physiology; SOFA, sequential organ failure assessment.
Logistic regression analysis
The multivariate non-conditioned logistic regression analysis was conducted with sepsis as a dependent variable, and heart rate, WBC, lymphocyte ratio, percentage of CD4+ T cells, CD4+PD-1+, CD8+PD-1+ and CD8+CTLA-4+, which were proven to be risk factors with statistical significances by above analysis, as independent variables. The results showed that heart rate (P = 0.022, EXP (B) = 1.200, 95% CI = 1.027-1.401), expression level of CD4+PD-1+ (P = 0.003, EXP (B) = 1.530, 95% CI = 1.159-2.018), and expression level of CD8+CTLA-4+ (P < 0.001, EXP (B) = 1.305, 95% CI = 1.133-1.503) might be risk factors for sepsis, while the percentage of CD4+ T cells (P < 0.001, EXP (B) = 0.574, 95% CI = 0.453-0.726) might be a protective factor for sepsis (Table 5).
Table 5.
Logistic regression analysis of the risk factors for sepsis
| Variables | Regression coefficient | Standard error | Wald value | P | EXP (B) | 95% CI | |
|---|---|---|---|---|---|---|---|
| Heart rate | 0.182 | 0.079 | 5.267 | 0.022 | 1.200 | 1.027 | 1.401 |
| WBC | -0.428 | 0.236 | 3.299 | 0.069 | 0.652 | 0.410 | 1.035 |
| Lymphocyte ratio | 0.134 | 0.143 | 0.877 | 0.349 | 1.144 | 0.865 | 1.515 |
| Percentage of CD4+ T cells | -0.556 | 0.121 | 21.269 | < 0.001 | 0.574 | 0.453 | 0.726 |
| CD4+PD-1+ | 0.425 | 0.141 | 9.040 | 0.003 | 1.530 | 1.159 | 2.018 |
| CD8+PD-1+ | 1.133 | 0.795 | 2.030 | 0.154 | 3.106 | 0.653 | 14.762 |
| CD4+CTLA-4+ | 0.266 | 0.072 | 13.647 | < 0.001 | 1.305 | 1.133 | 1.503 |
Note: WBC, white blood cell; EXP (B), exponential (B); 95% CI, 95% confidence interval.
Discussion
Our present results demonstrated that high expression level of CD4+PD-1+ and heart rate are potential risk factors for sepsis; while high percentage of CD4+ T cells is a possible protective factor for sepsis. The findings of flow cytometry in our present study showed that the expression level of CD4+PD-4+ in the patients with mild sepsis, severe sepsis or septic shock were higher as compared with healthy controls, suggesting that high expression level of PD-1 may serve as a pathophysiologic hallmark of sepsis. These in vitro outcomings strengthen the proposal that PD-1 pathway supplies a promising novel approach in the administration of sepsis. In addition, our results demonstrated that the percentage of CD4+ T cells in peripheral blood of the patients with septic shock was the lowest, and patients with severe sepsis, patients with mild sepsis, healthy controls, successively, further revealing that the PD-1 pathway may be critical in sepsis by regulating T cell co-stimulatory signal. However, no obvious correlations were observed between Tim-3 and CTLA-4 expressions and the progression of sepsis in our present study.
During the inflammatory response, T lymphocytes play an important role in suppression of immuneresponse, as demonstrated by the increase in number and functional enhancement following the onset of severe sepsis or septic shock [25]. A possible strategy for the improvement of host immunologic defenses is the application of agents that up-regulate adaptive immunity through the blockage of inhibitory receptors that are expressed on T lymphocytes, which has presented efficacy in many infectious models [10]. The activation of T cell is controlled by the expression of positive and negative co-stimulatory molecules preventing excessive function of T cell [26]. During inflammatory states, the expression of PD-1 is highly up-regulated causing inhibition of many T cell functions, such as proliferation, cytotoxic activity and cytokine production, and leading to T cell “exhaustion” [27,28]. These “exhausted” T cells are non-functional, prone to apoptosis, and are incapable of participating in effective immune responses, thus resulting in the chronic nature of viral infections [28]. Therefore, blocking PD-1 by using inhibitory antibodies has been demonstrated to restore the function of T cells, enhance antiviral responses in T cells, and decrease viral load in sepsis [29]. In line with our results, recent studies displayed that the over-expression of PD-1 on circulating T cells in sepsis patients is related to reduced proliferative capacity in T cells, and enhanced secondary nosocomial infections and mortality [1,30].
The results in our study also showed that heart rate in patients with mild sepsis, severe sepsis or septic shock were higher than that in the healthy controls, suggesting high heart rate may also serve as a pathophysiologic hallmark of sepsis. The monitoring of heart rate for early detecting sepsis, which applied mathematical methods to report the fold-increase in sepsis risk, provides independent information to laboratory tests and clinical findings, and reduced the mortality of very low birth weight infants with sepsis, in a large randomized trial, in neonatal intensive care unit (NICU) [31]. In addition, Douglas et al. have systematically assessed the application of heart rate for the screening of premature sepsis infants, documenting that the monitoring of heart rate characteristics is a validated risk marker for sepsis in the NICU [32]. Furthermore, Brown et al. have reported that increased variability of heart rate measured at 30-second intervals was associated with increased probability of successful early resuscitation of severe sepsis and septic shock after controlling for covariates [33].
In conclusion, PD-1 aggravates key immune responses consistent with promotion of T cell exhaustion in peripheral venous blood of patients with sepsis. High expression level of CD4+PD-1+ and heart rate are potential risk factors for sepsis; while high percentage of CD4+ T cells is a possible protective factor for sepsis. Consequently, the present results suggest that T cell exhaustion is a major etiology of immune dysfunction in sepsis, and the reversal of putative T cell exhaustion by utilizing anti-PD-1 provides promise in the treatment of sepsis.
Acknowledgements
We would like to acknowledge the reviewers for their helpful comments on this paper.
Disclosure of conflict of interest
None.
References
- 1.Chang KC, Burnham CA, Compton SM, Rasche DP, Mazuski RJ, McDonough JS, Unsinger J, Korman AJ, Green JM, Hotchkiss RS. Blockade of the negative co-stimulatory molecules PD-1 and CTLA-4 improves survival in primary and secondary fungal sepsis. Crit Care. 2013;17:R85. doi: 10.1186/cc12711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jawad I, Luksic I, Rafnsson SB. Assessing available information on the burden of sepsis: global estimates of incidence, prevalence and mortality. J Glob Health. 2012;2:010404. doi: 10.7189/jogh.02.010404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martin GS. Sepsis, severe sepsis and septic shock: changes in incidence, pathogens and outcomes. Expert Rev Anti Infect Ther. 2012;10:701–6. doi: 10.1586/eri.12.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, Sevransky JE, Sprung CL, Douglas IS, Jaeschke R, Osborn TM, Nunnally ME, Townsend SR, Reinhart K, Kleinpell RM, Angus DC, Deutschman CS, Machado FR, Rubenfeld GD, Webb S, Beale RJ, Vincent JL, Moreno R Surviving Sepsis Campaign Guidelines Committee including the Pediatric Subgroup. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med. 2013;39:165–228. doi: 10.1007/s00134-012-2769-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fan PC, Chang CH, Tsai MH, Lin SM, Jenq CC, Hsu HH, Chang MY, Tian YC, Hung CC, Fang JT, Yang CW, Chen YC. Predictive value of acute kidney injury in medical intensive care patients with sepsis originating from different infection sites. Am J Med Sci. 2012;344:83–9. doi: 10.1097/MAJ.0b013e3182373d36. [DOI] [PubMed] [Google Scholar]
- 6.Walkey AJ, Greiner MA, Heckbert SR, Jensen PN, Piccini JP, Sinner MF, Curtis LH, Benjamin EJ. Atrial fibrillation among Medicare beneficiaries hospitalized with sepsis: incidence and risk factors. Am Heart J. 2013;165:949–55. e3. doi: 10.1016/j.ahj.2013.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang Y, Li J, Lou J, Zhou Y, Bo L, Zhu J, Zhu K, Wan X, Cai Z, Deng X. Upregulation of programmed death-1 on T cells and programmed death ligand-1 on monocytes in septic shock patients. Crit Care. 2011;15:R70. doi: 10.1186/cc10059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tang L, Bai J, Chung CS, Lomas-Neira J, Chen Y, Huang X, Ayala A. Programmed cell death receptor ligand 1 modulates the regulatory T cells’ capacity to repress shock/sepsis-induced indirect acute lung injury by recruiting phosphatase SRC homology region 2 domain-containing phosphatase 1. Shock. 2015;43:47–54. doi: 10.1097/SHK.0000000000000247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang Y, Zhou Y, Lou J, Li J, Bo L, Zhu K, Wan X, Deng X, Cai Z. PD-L1 blockade improves survival in experimental sepsis by inhibiting lymphocyte apoptosis and reversing monocyte dysfunction. Crit Care. 2010;14:R220. doi: 10.1186/cc9354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chang K, Svabek C, Vazquez-Guillamet C, Sato B, Rasche D, Wilson S, Robbins P, Ulbrandt N, Suzich J, Green J, Patera AC, Blair W, Krishnan S, Hotchkiss R. Targeting the programmed cell death 1: programmed cell death ligand 1 pathway reverses T cell exhaustion in patients with sepsis. Crit Care. 2014;18:R3. doi: 10.1186/cc13176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJ, Robert L, Chmielowski B, Spasic M, Henry G, Ciobanu V, West AN, Carmona M, Kivork C, Seja E, Cherry G, Gutierrez AJ, Grogan TR, Mateus C, Tomasic G, Glaspy JA, Emerson RO, Robins H, Pierce RH, Elashoff DA, Robert C, Ribas A. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515:568–71. doi: 10.1038/nature13954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tang L, Bai J, Chung CS, Lomas-Neira J, Chen Y, Huang X, Ayala A. Active players in resolution of shock/sepsis induced indirect lung injury: immunomodulatory effects of Tregs and PD-1. J Leukoc Biol. 2014;96:809–20. doi: 10.1189/jlb.4MA1213-647RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anderson AC. Tim-3, a negative regulator of anti-tumor immunity. Curr Opin Immunol. 2012;24:213–6. doi: 10.1016/j.coi.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 14.Xiao T, Zhang L, Chen L, Liu G, Feng Z, Gao L. Tim-3 expression is increased on peripheral T cells from diffuse large B cell lymphoma. Tumour Biol. 2014;35:7951–6. doi: 10.1007/s13277-014-2080-0. [DOI] [PubMed] [Google Scholar]
- 15.Han G, Chen G, Shen B, Li Y. Tim-3: an activation marker and activation limiter of innate immune cells. Front Immunol. 2013;4:449. doi: 10.3389/fimmu.2013.00449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang L, Zhao C, Peng Q, Shi J, Gu G. Expression levels of CD28, CTLA-4, PD-1 and Tim-3 as novel indicators of T-cell immune function in patients with chronic hepatitis B virus infection. Biomed Rep. 2014;2:270–4. doi: 10.3892/br.2014.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pentcheva-Hoang T, Simpson TR, Montalvo-Ortiz W, Allison JP. Cytotoxic T lymphocyte antigen-4 blockade enhances antitumor immunity by stimulating melanoma-specific T-cell motility. Cancer Immunol Res. 2014;2:970–80. doi: 10.1158/2326-6066.CIR-14-0104. [DOI] [PubMed] [Google Scholar]
- 18.Winograd R, Byrne KT, Evans RA, Odorizzi PM, Meyer AR, Bajor DL, Clendenin C, Stanger BZ, Furth EE, Wherry EJ, Vonderheide RH. Induction of T-cell Immunity Overcomes Complete Resistance to PD-1 and CTLA-4 Blockade and Improves Survival in Pancreatic Carcinoma. Cancer Immunol Res. 2015;3:399–411. doi: 10.1158/2326-6066.CIR-14-0215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tarhini A. Immune-mediated adverse events associated with ipilimumab ctla-4 blockade therapy: the underlying mechanisms and clinical management. Scientifica (Cairo) 2013;2013:857519. doi: 10.1155/2013/857519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cao C, Ma T, Chai YF, Shou ST. The role of regulatory T cells in immune dysfunction during sepsis. World J Emerg Med. 2015;6:5–9. doi: 10.5847/wjem.j.1920-8642.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hutchins NA, Unsinger J, Hotchkiss RS, Ayala A. The new normal: immunomodulatory agents against sepsis immune suppression. Trends Mol Med. 2014;20:224–33. doi: 10.1016/j.molmed.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, Cohen J, Opal SM, Vincent JL, Ramsay G International Sepsis Definitions Conference. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Intensive Care Med. 2003;29:530–8. doi: 10.1007/s00134-003-1662-x. [DOI] [PubMed] [Google Scholar]
- 23.Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, Cohen J, Opal SM, Vincent JL, Ramsay G Sccm/Esicm/Accp/Ats/Sis. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med. 2003;31:1250–6. doi: 10.1097/01.CCM.0000050454.01978.3B. [DOI] [PubMed] [Google Scholar]
- 24.van Ruler O, Kiewiet JJ, Boer KR, Lamme B, Gouma DJ, Boermeester MA, Reitsma JB. Failure of available scoring systems to predict ongoing infection in patients with abdominal sepsis after their initial emergency laparotomy. BMC Surg. 2011;11:38. doi: 10.1186/1471-2482-11-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chaudhry A, Rudensky AY. Control of inflammation by integration of environmental cues by regulatory T cells. J Clin Invest. 2013;123:939–44. doi: 10.1172/JCI57175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen L, Flies DB. Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat Rev Immunol. 2013;13:227–42. doi: 10.1038/nri3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Okazaki T, Chikuma S, Iwai Y, Fagarasan S, Honjo T. A rheostat for immune responses: the unique properties of PD-1 and their advantages for clinical application. Nat Immunol. 2013;14:1212–8. doi: 10.1038/ni.2762. [DOI] [PubMed] [Google Scholar]
- 28.Penaloza-MacMaster P, Kamphorst AO, Wieland A, Araki K, Iyer SS, West EE, O’Mara L, Yang S, Konieczny BT, Sharpe AH, Freeman GJ, Rudensky AY, Ahmed R. Interplay between regulatory T cells and PD-1 in modulating T cell exhaustion and viral control during chronic LCMV infection. J Exp Med. 2014;211:1905–18. doi: 10.1084/jem.20132577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Binder DC, Schreiber H. Dual blockade of PD-1 and CTLA-4 combined with tumor vaccine effectively restores T-cell rejection function in tumors--letter. Cancer Res. 2014;74:632. doi: 10.1158/0008-5472.CAN-13-2216. discussion 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang X, Venet F, Wang YL, Lepape A, Yuan Z, Chen Y, Swan R, Kherouf H, Monneret G, Chung CS, Ayala A. PD-1 expression by macrophages plays a pathologic role in altering microbial clearance and the innate inflammatory response to sepsis. Proc Natl Acad Sci U S A. 2009;106:6303–8. doi: 10.1073/pnas.0809422106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fairchild KD, O’Shea TM. Heart rate characteristics: physiomarkers for detection of late-onset neonatal sepsis. Clin Perinatol. 2010;37:581–98. doi: 10.1016/j.clp.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lake DE, Fairchild KD, Moorman JR. Complex signals bioinformatics: evaluation of heart rate characteristics monitoring as a novel risk marker for neonatal sepsis. J Clin Monit Comput. 2014;28:329–39. doi: 10.1007/s10877-013-9530-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brown SM, Tate MQ, Jones JP, Kuttler KG, Lanspa MJ, Rondina MT, Grissom CK, Mathews VJ. Coefficient of Variation of Coarsely Sampled Heart Rate is Associated With Early Vasopressor Independence in Severe Sepsis and Septic Shock. J Intensive Care Med. 2015;30:420–5. doi: 10.1177/0885066614523536. [DOI] [PMC free article] [PubMed] [Google Scholar]


