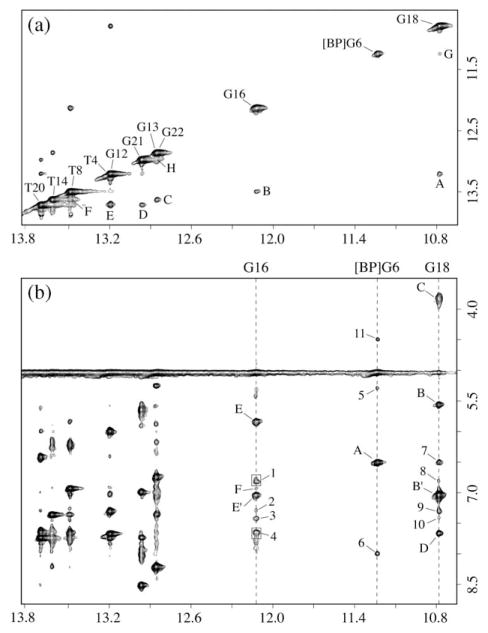
Figure 3.
Expanded NOESY (200 ms mixing time) contour plots for the 10R-(−)-trans-anti-[BP]G adduct in the meC-[BP]G-C sequence context at the 11-mer duplex level. Spectra are in 100 mM NaCl, 10 mM phosphate, H2O, pH 6.8 at 0 °C. (a) NOEs between imino protons, with the cross-peaks labeled A to H, which are assigned as follows: A, T4(NH3)-G18(NH1); B, T8(NH3)-G16(NH1); C, T14(NH3)-G13(NH1); D, T20(NH3)-G21(NH1); E, T4(NH3)-T20(NH3); F, T8(NH3)-T14(NH3); G, [BP]G6(NH1)-G18(NH1); H, G21(NH1)-G22(NH1). (b) NOEs between imino protons (10.5–14.0 ppm) of dG16, [BP]dG6, and dG18, and amino and non-exchangeable protons (3.5–8.5 ppm), with NOE cross-peaks between DNA protons labeled A to F and NOE cross-peaks between carcinogen and DNA protons labeled 1–11. Cross-peaks A to F are assigned as follows: A, [BP]G6(NH1)-[BP]G6(NH2)/BP(H10); B,B′, G18(NH1)-[Me]C5(NH2); C, G18(NH1)-G18(NH2); D, G18(NH1)-A19(H2); E,E′, G16(NH1)-C7(NH2); F, G16(NH1)-A15(H2). Cross-peaks 1–10 are assigned as follows: 1, G16(NH1)-BP(H4,H5); 2, G16(NH1)-BP(H6); 3, G16(NH1)-BP(H3); 4, G16(NH1)-BP(H1,H2); 5, [BP]G6(NH1)-[Me]C5(H1′); 6, [BP]G6(NH1)-BP(H11); 7, G18(NH1)-[BP]G6(NH2)/BP(H10); 8, G18(NH1)-BP(H4,H5); 9, G18(NH1)-BP(H6); 10, G18(NH1)-BP(H3); 11, [BP]G6(NH1)-BP(H9).