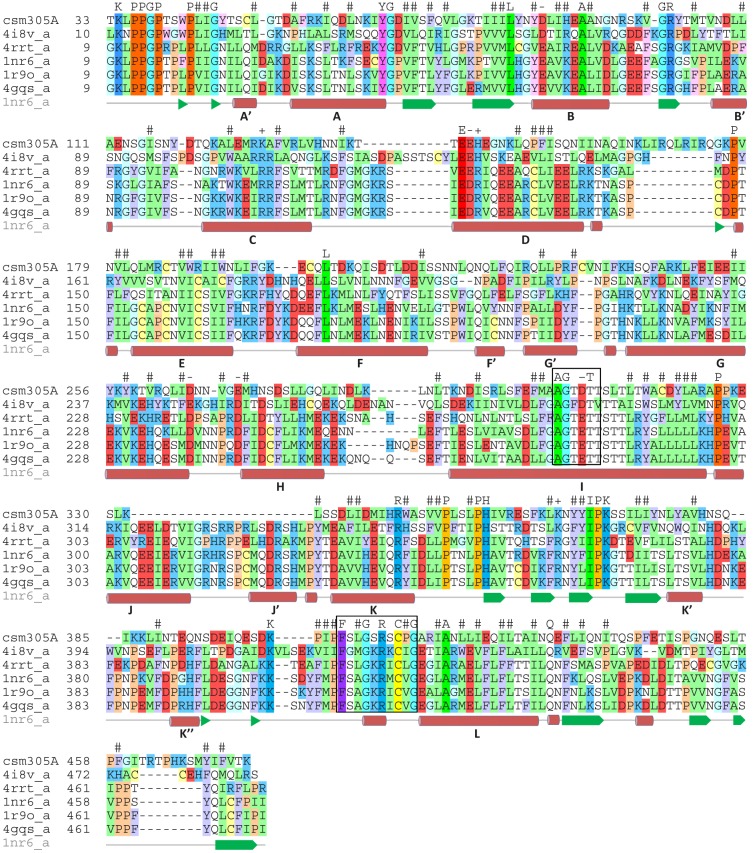
Fig 2. Comparison of Schistosoma mansoni CYP450 protein (Sman) with CYP450 proteins from other species.
Multiple alignment of CYP450 proteins from S. mansoni (csm305A); rabbit CYP450 2C5 (1nr6_a); human CYP450 2C9 (1r9o_a); human CYP450 2C19 (4gqs_a); human CYP450 1A1 (4i8v_a); and human CYP450 2b6 (4rrt_a). The residues are shown in one letter code and colored by type: red- negatively charged, blue—positively charged, yellow—Cys, green—hydrophobic, cyan—Gly, ochre—Pro, purple—aromatic. The residues are shown in brighter colors for conserved positions. The ‘P450-signature’ sequence, which forms a channel for electron transfer, and the CYP450 consensus motif responsible for heme-binding and interaction with molecular oxygen and the relevant substrates are boxed. Predicted helices in the secondary structure based on homology modelling of SmCYP450 are indicated by the bold letters A-L based on rabbit CYP450 2C5 [38].