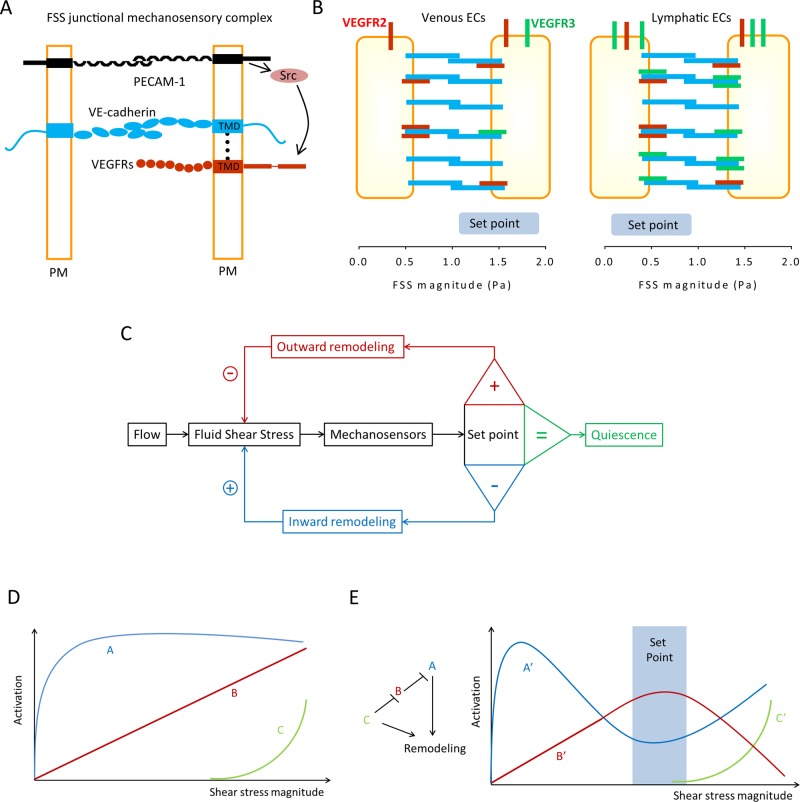
FIGURE 1:
Control of vascular remodeling by fluid shear stress sensing. (A) Endothelial mechanosensitive junctional complex. Conceptual model for the assembly of the junctional mechanosensory complex. VEGF receptors (both VEGFR2 and VEGFR3) and VE-cadherin associate through their transmembrane domains (TMDs) within the plasma membrane (PM). FSS triggers force on PECAM-1, which leads to activation of a src family kinase (Src), which phosphorylates and activates VEGFRs. (B) Blood vs. lymphatic ECs. Higher VEGFR3 (green) expression in lymphatic ECs increases their sensitivity to FSS, resulting in a lower FSS set point in lymphatic compared with blood ECs. VEGFR2 is in red. (C) Classical control theory. In this model, ECs sense FSS magnitude and compare it with a pre-existing value, the FSS set point. Deviations from this value activate remodeling mechanisms to alter vessel diameter and return FSS to the steady-state level. This model is unlikely to describe a biological mechanism. (D) Biological pathways. We speculate that a more realistic mechanism for the FSS set point requires several mechanosensitive elements, denoted A, B, and C, that have different sensitivities. Pathway A is activated and reaches a maximum at low FSS, while the other(s) may require higher FSS. (E) Integration to determine the set point. The outputs from A, B, and C are denoted A′, B′, and C′. These pathways would be organized into a network such that B′ is maximal at an intermediate FSS level, the FSS set point (blue rectangle). B′ stabilizes the vessel and inhibits remodeling. The ratio of A′ and B′ determine the direction of remodeling, where high A′/low C′ gives inward remodeling and high A′/high C′ gives outward remodeling. This model is highly hypothetical and is meant only to illustrate the general class of theories that might explain the set point.