Figure 6. Expression of a catalytically active IRE1α C-terminal deletion mutant does not interfere with the angiogenic and invasive processes.
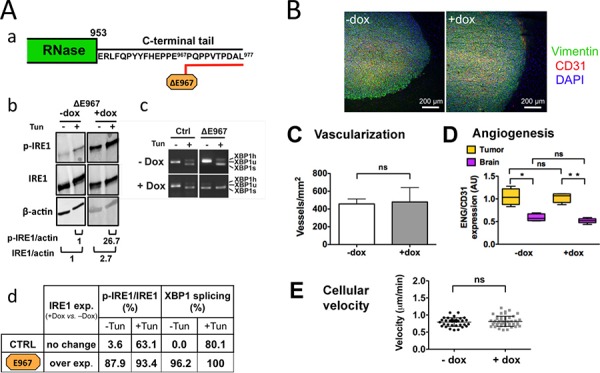
Cells were designed to stably express an IRE1α protein truncated at its C-terminus by 10 aminoacids. Aa. C-terminal tail deletion of IRE1α; desing of the ΔE967 mutant. Cells were grown for 7 days in culture with or without doxycycline (+Dox and –Dox, respectively) and were analyzed for transgene expression and inhibition of XBP1 mRNA splicing following incubation for 2 h with or without tunicamycine (Tun). Ab. Western blot analysis. Quantification of p-IRE1α and total IRE1α proteins were normalized to actin. Ac. Measure of XBP1-splicing. Ad. Measures in percent (+Dox vs. –Dox) of IRE1α kinase and RNase activities in cells expressing either the wild-type IRE1α protein (CTRL) or the IRE1α-ΔE967 transgene product. Relative values of IRE1α autophosphorylation and of XBP1 splicing were determined as in figure 1. B. Coronal sections of U87-ΔE967 gliomas grown for 28 days in the mouse brain in the presence or absence of doxycycline. IHC labeling of vimentin, CD31 and DAPI was carried out as in figure 3. C-D. Vascularization of glioma tissues and of the brain parenchyma was expressed as the total number of blood vessels (CD31 labeling). Angiogenesis was quantified by measuring the number of proliferative blood vessels (ENG vs. CD31 labelings; see figure 3.) E. Expression of the IRE1α ΔE967 mutant did not increase cell motility and chemotaxis (see also figure 5D–5E).
